Galectin-3 (Gal-3) belongs to a lectin family, acting as a galactoside-binding protein involved in many biological processes, such as controlling cell–cell and cell–matrix interactions, adhesion, proliferation, apoptosis, pre-mRNA splicing, immunity and inflammation.
- Galectin-3
- atherosclerosis
- cardiovascular diseas
1. Introduction
The galectin family includes fifteen members, classified into three groups according to their structures: proto, chimera and tandem repeat types. Gal-3 is the only member characterized by a chimeric structure (Figure 1), showing a carbohydrate recognition domain and a non-lectin N-terminal domain promoting oligomerization [1]. Indeed, Gal-3 is the only galectin able to form pentamers. The N-terminal domain of Gal-3 contains a phosphorylation site at serine 6 regulating its nuclear localization and reducing the affinity for its ligands [2][3].
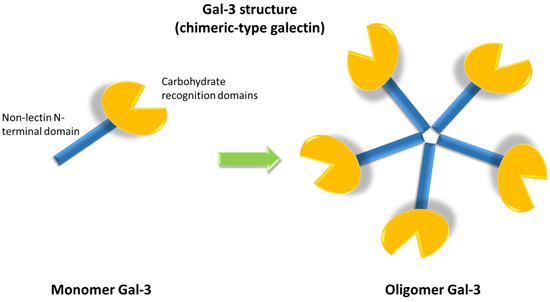
Figure 1. Galectin-3 (Gal-3) chimeric structure characterized by a carbohydrate recognition domain and a non-lectin N-terminal domain that promotes oligomerization, allowing for the formation of pentamers.
The region between the carbohydrate recognition domains and the N-terminal domain forms a collagen-like sequence of about 100 amino acids and contains the collagenase cleavable H-domain [114].
The protein was originally identified in murine peritoneal macrophages and named MAC-2 because of its capacity to identify a macrophage sub-population [125]. The protein was also identified in different pathways and independently described as IgE binding protein, L-29, CBP30 or CBP35 [136].
In humans, Gal-3 is a 35 kDa protein coded by the LGALS3 gene located on chromosome 14 [147]. Human Gal-3 is expressed by macrophages during phagocytosis and affects the differentiation and growth of various immune cells, which play a role in angiogenesis. Moreover, Gal-3 is able to induce migration and proliferation of endothelial cells [158].
The protein acts during embryogenesis and is expressed in principal and intercalated cells of the collecting duct of the kidney as well as in later stages of nephrogenesis [169]. Gal-3 is mainly present in the cytoplasm, but it can also be found in the nucleus, on the cell surface and in the extra-cellular environment [1710].
The different locations of Gal-3 account for its various functions:
33]. The subsequent inflammatory response involves a massive participation of monocytes and macrophages altering the structure of the intima and media layers of the wall [3][4034]. Endothelial cells become activated and SMCs actively proliferate, producing extracellular matrix. The narrowing of the artery lumen leads to a decreased blood flow that can be completely arrested in case of a thrombus formation on the plaque surface, causing acute events such as acute myocardial infarction (AMI) or stroke. The involvement of many inflammatory markers in the atherosclerotic process has been investigated over the years [4236][4337][4438][4539] and a potential role of Gal-3 as a mediator for atherosclerosis emerged [4640].
By studying samples from young adult trauma victims, a higher concentration of Gal-3 was detected in foam cells and macrophages compared to the vascular smooth muscle cells (VSMC) of atherosclerotic lesions [4741]. Gal-3 also regulates chronic inflammation at the cardiovascular level, promoting osteogenic differentiation of VSMCs that favours macrocalcifications in atherosclerotic plaques [1][4842][4943].
Gal-3 was expressed not only by inflammatory cells in unstable plaque regions, but also by VSMCs in fibrosis and particularly of sheet-like/lamellated calcification areas, indicating a more stable pattern [5044]. Based on these data, the authors proposed a molecular mechanism by which Gal-3 modulates not only inflammation, but also vascular osteogenesis, with its expression necessary to acquire a complete osteoblast-like phenotype by VSMCs. Another study highlighted that Gal-3 over-expression in VSMCs induced collagen type I increased synthesis [5044], supporting its role as a remodelling factor of the vascular wall [5145].
In patients with high-grade carotid stenosis, low Gal-3 intra-plaque concentration was correlated with clinically and ultrasonically defined unstable human carotid plaques, suggesting a possible use of Gal-3 in identifying carotid plaques prone to rupture and cerebral embolization [5246].
2.2. In Vivo Studies
The possibility of carrying out in vivo studies, with the involvement of animal models, provided further insights into the direct role of Gal-3 in the atherosclerotic process [4943][5347][5448][5549][5650][5751][5852][5953][6054].
Table 1 reports the in vivo studies performed on different animal models. Using the Apoe knock-out mice as atherosclerotic-prone animals, a study revealed an increased expression of Gal-3 within the lesion [5448]. Most of the studies inhibiting Gal-3 action by knock-out or by a direct inhibitor such as Modified Citrus Pectin (MCP) demonstrated a decreased formation of atherosclerotic lesions but increased lesions with vulnerable features, i.e., lesions with microcalcification, diffused inflammation and low collagen content. Taken together, these results highlight the conflicting aspects of Gal-3 involvement in atherogenesis and its complications.
Table 1. Main in vivo studies on the direct role of Gal-3 in the atherosclerotic process.
| Papers | Experimental Model | Effects |
|---|---|---|
| Menini et al., 2013 [4943] | Lgals3−/− | No osteogenic differentiation of vascular smooth muscle cells resulting in ↓ plaque stability |
| Iacobini et al., 2009 [5347] | Lgals3−/− mice | ↑ Lesion area and length; complex lesions and extensive inflammation at the aortic sinus. |
| Lee et al., 2013 [5448] | Apoe−/− mice | ↑ Gal-3 expression within atherosclerotic plaques proportionally to the extent of plaque inflammation; Gal-3 colocalization with plaque macrophages’ distribution. |
| Lu et al., 2017 [5549] | Apoe−/− mice + inhibitor of Gal-3 (MCP) | ↓ size of atherosclerotic lesions, ↓number of macrophages and smooth muscle cells in the lesions, ↓endothelial injury. |
| Nachtigal et al., 2008 [5650] | Lgals3−/−:Apoe−/− mice | ↓ Atherosclerotic lesions ↓ perivascular inflammatory infiltrates and mast cells. |
| MacKinnon et al., 2013 [5751] | Lgals3−/−:Apoe−/− mice | ↓ Atherosclerotic lesions. |
| Sun et al., 2019 [5852] | Apoe−/− mice + Gal-3 silencing | ↑ microcalcification in the plaque. |
| Di Gregoli et al., 2020 [5953] | Lgals3−/−:Apoe−/− mice | Altered plaque composition, ↓ collagen content, ↑ necrotic area, ↑ invasive capacity of macrophages, ↑ expression of proinflammatory genes. |
| Mmp12−/−:Apoe−/− mice | ↓ plasma levels of soluble Gal-3; ↑ Gal-3 expression within plaques. |
-
cytoplasm: regulation of the cell cycle, inducing proliferation and anti-apoptotic effects, through its interaction with survival-associated proteins [7][11][12][13][14][15][16].
-
nucleus: regulation of gene transcription and promotion of pre-mRNA splicing [17][15][16].
-
cell- surface: involved in lattice assembly, a multi-dimensional organization consisting of Gal-3 in its different forms and several types of saccharide ligands [6][18]. The main functions of lattice are regulation of diffusion, compartmentalization and endocytosis of plasma membrane glycoproteins and glycolipids, selection, activation and arrest of T-cells, signaling of receptor kinase and membrane receptors [19].
-
extracellular environment: binding to different cell surface and extracellular matrix glycans, in order to induce cell adhesion, migration, and growth regulation, mainly pro-apoptotic effects [4][20][21][22].
-
cytoplasm: regulation of the cell cycle, inducing proliferation and anti-apoptotic effects, through its interaction with survival-associated proteins [14][18][19][20][21][22][23].
-
nucleus: regulation of gene transcription and promotion of pre-mRNA splicing [6][22][23].
-
cell- surface: involved in lattice assembly, a multi-dimensional organization consisting of Gal-3 in its different forms and several types of saccharide ligands [13][24]. The main functions of lattice are regulation of diffusion, compartmentalization and endocytosis of plasma membrane glycoproteins and glycolipids, selection, activation and arrest of T-cells, signaling of receptor kinase and membrane receptors [25].
-
extracellular environment: binding to different cell surface and extracellular matrix glycans, in order to induce cell adhesion, migration, and growth regulation, mainly pro-apoptotic effects [11][26][27][28].
Due to its multifunctional role, the protein also plays a relevant role in many different clinical conditions and diseases, ranging from asthma to cancer, gastritis, obesity and renal diseases, as reported in Figure 2 and recently reviewed [617].
Figure 2. Schematic representation of main roles of Gal-3 in several physiologic and pathogenetic mechanisms.
2. Molecular Mechanisms
2.1. Gal-3 in Inflammation and Atherosclerosis
As a potent inflammatory protein, Gal-3 contributes to the initiation and amplification of the inflammatory response, being related to both acute and chronic inflammation [2923].
In the acute inflammatory response, Gal-3 acts as a promoter of chemoattraction of monocytes/macrophages [3024], neutrophil clearance [3125], opsonization of apoptotic neutrophils [3226], and mast cell degranulation [3327]. Moreover, in macrophages and epithelial cells, Gal-3 binds galactoside-associated membrane remnants from vacuoles produced by some intravacuolar bacteria to actively evade the phagolysosomal pathway of the host [3428][3529][3630]. This binding allows to target damaged vesicles for autophagy through the recruitment of autophagy adaptor proteins.
Gal-3 has a wide distribution in primary and secondary lymphoid organs and in circulating immune cells [3731]. In addition, Gal-3 is up-regulated in both CD4+ and CD8+ activated murine T lymphocytes but not in resting T cells. Gal-3 inhibition by an antisense oligonucleotide suppresses the proliferation of T cells [3832]. This evidence suggests an active role of Gal-3 in inflammation promotion.[3933][4034]
Although inflammation is essential for tissue healing, constant and sustained inflammation may lead to tissue damage and organ failure. Atherosclerosis is a complex inflammatory process initiated by altered cellular permeability of the arterial walls and focal sub-endothelial accumulation of LDL cholesterol (LDL-C), leading to the development of atherosclerotic plaques characterized by inflammation and oxidation [4135][39
2.3. Gal-3 in Cardiac Fibrosis and Heart Failure
Cardiac fibrosis is a tissue repair mechanism caused by a progressive accumulation of extracellular matrix in response to injury, inflammation or stress. The process leads to an impaired tissue-repair [6054], causing tissue and organ scarring, injury, and reduction or loss of function [61,62][43][44].
Gal-3 expression is associated with abundant macrophages, increased fibroblast activity, accumulation of extracellular matrix [45] [63] and with collagen production in the myocardium [64][46]. The protein is also expressed by fibroblasts and macrophages following stressful events [60,65,66][42][47][48].
Gal-3 increase was evidenced in rat models after myocardial infarction, showing a later peak in non-infarcted myocardium involved in cardiac remodelling [67][55]. Rats intrapericardially infused with Gal-3 reported an enhanced macrophage and mast cell infiltration, increased cardiac interstitial and perivascular fibrosis, and cardiac hypertrophy [68][56].
After its activation, Gal-3 forms a lattice complex entrapping TGFβ on the cell surface that gives a prolonged signal of fibrotic development [69,70][57][58]. These signalling factors, together with mechanical stress, transform quiescent fibroblasts into active collagen-producing myofibroblasts [71][59].
Gal-3 inhibition by MCP is effective in decreasing both the ischemic area and the fibrotic remodeling in a rat model of ischemia/reperfusion [72][60]. Similarly, silencing of Gal-3 in cardiomyocytes isolated from rats and treated to simulate ischemia/reperfusion, resulted in a decreased apoptosis thanks to its interaction with bcl-2 [73][61].
Closely related to the fibrotic process, Heart Failure (HF) is a clinical syndrome caused by a structural/functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intra-cardiac pressure at rest or during stress, leading to typical symptoms and signs (e.g., breathlessness, fatigue, elevated jugular venous pressure, pulmonary crackles and peripheral oedema) [74][62]. The two major pathophysiological processes contributing to HF development are inflammation and fibrosis, which affect tissue architecture, electrical conduction, mechano-electrical coupling and force generation by cardiomyocytes [75][63]. The prevalence of HF is globally increasing, mainly due to population aging [64] [76] and the more successful treatment of cardiovascular diseases preceding HF, such as myocardial infarction, myocarditis and hypertension [77][65].
Gal-3 is involved in the pathophysiology of HF [66] [78] mainly because of its role in cardiac ventricular remodeling [71,77][59][65]. Normally, Gal-3 expression in the heart is low, whereas its synthesis and secretion increase in HF [63,79,80][45][67][68]. Gal-3 initially plays a protective role in the heart through its anti-apoptotic and anti-necrotic functions, while the prolonged expression of this protein leads to fibrosis and adverse remodeling of the damaged tissue [78][66]. Gal-3 binding sites are mainly localized in the myocardial matrix, fibroblasts and macrophages. At the site of injury, Gal-3 is secreted into the extracellular space and activates resting fibroblasts into matrix-producing fibroblasts. The role of Gal-3 in fibroblast activation involves up-regulation of the cytoskeletal proteins, the synthesis of new matrix components such as type I collagen and the inhibition of extracellular matrix component degradation down-regulating matrix metalloproteinases [78][66]. Moreover, another study showed that Gal-3 infusion caused myocardial fibrosis, which was neutralized by an anti-fibrotic agent, suggesting that Gal-3 may be involved in the development and resolution of fibrosis [81][69].
In a rat model of hypertensive HF, recombinant Gal-3 infusion into the pericardial sac for 4 weeks induced excessive collagen deposition and cardiac dysfunction, which is likely to develop into HF [63][45].
Despite the fact that Gal-3 cardiac levels were increased in hearts from different mouse models of heart disease, Nguyen et al. demonstrated that Gal-3 circulating levels were high in mice with fibrotic cardiomyopathy, whereas normal levels were observed in the dilated cardiomyopathy mouse model [82][70]. The authors also observed that β-adrenoceptor activation is responsible for increased Gal-3 levels in both cardiac tissue and plasma.
2.4. Gal-3 and Other Cardiovscular Diseases
Atrial fibrillation (AF) is the most frequent arrhythmia and it is associated with structural, electrical, genomic and hormonal atrial remodeling [79][67]. More recently, the concept of fibrotic atrial cardiomyopathy was introduced—defined as a progressive invasion of atrial myocardium by fibrosis, which favors initiation and maintenance of AF [83][71]. A direct role of Gal-3 in AF was demonstrated by its action in: (i) promoting the retention of cytokine receptors on the atrial myofibroblast membrane, through entrapment within Gal-3 lattice [70][58]; (ii) interaction of the extracellular pentameric Gal-3 with molecules, such as TGF-β, which could contribute to initiate fibrogenesis [79][67]; (iii) promoting nuclear translocation of transcription factors such as β-catenin. In the nucleus, β-catenin induces the activation of transcription factors promoting collagen transcription [84][72].
After an experimentally induced stroke in mice, a high number of Gal-3 positive cells in the brain resulted, which was associated with a smaller infarct size and a better functional outcome, highlighting the contribution of Gal-3 to postischemic brain remodeling [85][73].
A critical role in venous thrombosis was also reported for Gal-3 and Gal-3–binding protein, which interact with the thrombus–vein wall interface contributing to thrombus formation through proinflammatory, IL-6–dependent mechanisms [86][74].
A recent paper suggested the involvement of Gal-3 in autoimmune myocarditis; in fact, Gal-3 knock-out mice developed more severe myocarditis and heart hypertrophy than wild-type mice upon induction of experimental autoimmune myocarditis [87][75].
3. Galectin-3 as a Circulating Marker
Biomarkers are objectively measured characteristics that can be used as indicators of normal biological or pathogenic processes [88][76] and may be useful for improving diagnosis, prognosis and risk stratification of cardiovascular diseases, including some genetic conditions [89,90,91,92,93,94,95][77][78][79][80][81][82][83]. A recent study established the upper reference limit (URL) of Gal-3 in a population of healthy subjects, identifying age as an important biological variable that affects Gal-3 concentration, suggesting multiple diagnostic cut-offs related to the different age groups [96][84].
To date, Gal-3 measurement is recommended by the 2017 Guidelines of the American Heart Association for risk stratification and prognosis evaluation of patients with HF [97][85]. Besides this application, Gal-3 levels have been investigated as biomarkers of several CVDs, in particular, in cardiovascular diseases initiated and stimulated by inflammation [98][86]. Recently, a possible role of Gal-3 as a marker of therapy-induced cardiotoxicity was also investigated; however, no association was found [99][87].
3.1. Atherosclerotic Plaques
Several studies evaluated the role of Gal-3 plasma levels as biomarkers of atherosclerosis highlighting correlations with plaque instability. For example, a study on patients with coronary artery disease (CAD) showed that unstable patients had higher plasma Gal-3 levels compared to the stable ones [100][88]. Moreover, the authors found a trend of correlations between plasma Gal-3 levels and the number of compromised vessels. This study further validates the association of Gal-3 with macrophage activation and monocyte attraction, thereby supporting its possible use as a biomarker of atherosclerotic plaques and of their destabilization.
Hypercholesterolemia is a well-known risk factor for atherosclerosis development, and the most severe form, the homozygous familial hypercholesterolemia, is associated with very early CVDs [101,102][89][90]. Despite being invasive, lipoprotein apheresis is one of most effective treatments of severe hypercholesterolemia and has been shown to contribute to the decrease in circulating Gal-3 levels in humans, enhancing the benefits from this treatment [103][91].
High levels of Gal-3 have been associated with high-grade carotid atherosclerosis [104,105][92][93]. In the Atherosclerosis Risk in Communities (ARIC) study, patients in the fifth quintile according to Gal-3 concentration showed a higher cIMT in comparison with patients in the first quintile [104][92]. Our research group recently demonstrated that high plasma concentrations of Gal-3 are reliable biomarkers of advanced atherosclerotic plaque as they are associated with plaque presence independent of age, sex, LDL-cholesterol levels and a previous AMI [105][93].
3.2. Atherosclerotic Cardiovascular Diseases
Gal-3 concentration and carotid intima-media thickness (cIMT) values emerged as independent predictive indicators of mortality risk in patients after MI during mid-term follow up [106][94].
Among a large population of 5805 elderly (mean age 69 years), low Gal-3 levels were a good negative risk marker for cardiovascular events, being associated with a low incidence of CVD events at a short-term follow-up. Interestingly, for risk assessment Gal-3 levels are even more relevant than the absence of carotid plaque evaluated by ultrasound [107][95].
As to patients undergoing coronary angiography for CAD, during long-term follow-up, cardiovascular deaths occurred more commonly in the high Gal-3 tertile, suggesting that plasma Gal-3 level is an independent predictor of cardiovascular mortality in patients with CAD [108][96]. In addition, a positive correlation between serum Gal-3 levels and CAD severity was also reported in another study [109][97].
In patients with CAD after AMI, a significant decrease in Gal-3 concentration was observed in subjects without acute events during follow-up observation [110][98].
High Gal-3 levels measured at the time of incident AMI were found to be an independent predictor of mortality, suggesting that measures of Gal-3 may be used for risk stratification post-AMI [111][99]. Furthermore, in post-myocardial infarction patients, it was shown that plasma Galectin-3 levels predict a high risk of deleterious vascular dysfunctions at 6 months [112][100].
A recent study highlighted that the association of high Gal-3 levels with cardiovascular events could be influenced by the presence of type-2 diabetes. In fact, Gal-3 plasma levels were associated with cardiovascular events in CAD patients with diabetes, whereas NT-proBNP in patients without diabetes [113][101].
A significant prognostic value of serum Gal-3 has also been reported for predicting severe abdominal aortic calcification in patients with end-stage renal disease undergoing hemodialysis [114][102].
High serum Gal-3 levels were associated with the presence of acute ischemic stroke (AIS) and are correlated with AIS severity and infarction volume [115][103]. Moreover, serum Gal-3 levels were significantly higher in patients with a poor outcome. Similarly, higher Gal-3 serum levels were found in patients with large artery atherosclerotic stroke compared to controls. In addition, an independent association was evidenced between Gal-3 levels and unfavorable outcomes after ischaemic stroke [116][104].
The increase in Gal-3 plasma levels in patients who underwent carotid endarterectomy were also associated with increased incidence of postoperative stroke [117][105]. In particular, in women, Gal-3 was a predictor of postoperative stroke even after correction for traditional risk factors, suggesting a potential role of the protein as a marker to predict postoperative cerebrovascular ischemic events.
As to atherosclerosis in other artery regions, a different distribution of Gal-3 was observed in normal arteries and arteries from patients with PAD. Indeed, Gal-3 was expressed in the adventitia in normal arteries, while in the arteries of PAD patients, Gal-3 was expressed mainly in the media and, to a lesser amount, in the adventitia and the intima. Moreover, serum Gal-3 levels were higher in PAD patients than in the control group, correlating well with other circulating markers of inflammation or oxidative stress [118][106].
Gal-3 concentrations were significantly associated with increased cardiovascular mortality risk in patients with peripheral artery disease (PAD) followed-up for 5 years [119][107].
Recently, higher levels of Gal-3 measured in 9851 ARIC Study participants free of PAD at baseline were significantly associated with an elevated risk of PAD and critical limb ischemia over 17.4 years of follow-up. This association was independent of traditional risk factors, including levels of C-reactive protein levels, suggesting that Gal-3 may enhance the prediction of incident PAD [120][108].
3.3. Heart Failure
The Gal-3 serum level has been approved as a diagnostic marker for risk stratification and prognosis evaluation of HF patients according to the 2017 ACC/AHA/HFSA Guidelines for the Management of HF [121][109]. Although the role of BNP and NT-pro-BNP for HF diagnosis and prognosis is indisputable, Gal-3 levels can improve patient management, providing additional information about possible risks of further hospitalization and death. A possible use of Gal-3 as a biomarker in HF was widely investigated and recently reviewed [77,122][65][110].
The threshold of 17.8 ng/mL of Gal-3 serum levels is usually considered to discriminate between HF patients at low and high risk for clinical complications [123][111]. Based on this cut-off, a pooled data analysis carried out in patients with HF (n = 902) enrolled in three cohorts (COACH, PRIDE and UMD H-23258) showed that plasma Gal-3 concentrations can be useful in HF hospitalized patients for near-term rehospitalization prediction [124][112].
The Valsartan Heart Failure Trial 2013 (n = 1650) reported that Gal-3 increase in patients with symptomatic HF was independently associated with worse outcomes [125][113]. A meta-analysis of 18 studies, involving 32,350 participants among the general population and patients with HF found a correlation between elevated plasma Gal-3 and risk of all-cause mortality, CVD mortality, and HF [126][114]. Conversely, a study performed in 1161 patients enrolled in the RELAX-AHF trial did not find an independent association of Gal-3 serum levels with cardiovascular mortality within 180 days of hospitalization for acute HF [127][115]. A recently published meta-analysis evaluated the prognostic role of Gal-3 serum levels in 7057 patients with acute HF [128][116], confirming that higher serum levels may be associated with poor prognosis in such patients.
Gal-3 may provide additional information in HF prognosis and risk stratification since the combination of biomarkers could be more informative than single biomarkers, as reported in the Scientific Statement from the American Heart Association [97][85]. The additional prognostic value of Gal-3 was demonstrated in the HF-ACTION study on a cohort of about 900 ambulatory patients with HF [129][117]. In this study, patients with high levels of both NT-proBNP and Gal-3 showed a Hazard Ratio of 2.19 for hospitalization at 4 years of follow-up compared to patients with low levels of both markers. In addition, a modest correlation of Gal-3 and NT-proBNP levels was observed, suggesting that these two markers usually increase simultaneously. However, in this study, the prognostic role of Gal-3 levels turns out to be inconsistent in a multivariate analysis including NT-proBNP levels, probably as a consequence of the above-mentioned correlation. Other studies demonstrated that the predictive value of Gal-3 is independent of NT-proBNP levels, age, gender and estimated glomerular filtration rate (eGFR) [130][118]. Decreased eGFR values are an additional risk factor in HF patients, often leading to re-hospitalization. Unfortunately, natriuretic peptide levels are increased in patients with low eGFR independently of the presence of HF. A recent review highlighted the different biomarkers that can be used for HF prognosis in patients with chronic kidney disease, indicating that Gal-3 could be more useful than NT-proBNP being less influenced by eGFR [131][119]. In patients with chronic kidney disease without signs of HF at recruitment, high Gal-3 levels were associated with early symptomatic changes of HF, although with a hazard ratio lower than that of growth differentiation factor-15 (GDF-15) [132][120].
The study of a large population of subjects free of HF (22,756 participants) followed for more than 12 years revealed a moderate contribution of Gal-3 in the prediction of HF development only in women [133][121]. In addition, differently from NT-proBNP, Gal-3 circulating levels were not associated with measures of cardiac mechanics in elderly subjects, indicating that Gal-3 cannot be used as a biomarker of pre-clinical heart failure [134][122]. On the other hand, in a community-based cohort, elevated plasma levels measured at midlife of Gal-3 are not only associated with incident heart failure, but also with incident coronary heart disease, ischemic stroke, and total mortality [135][123].
Taken together, this evidence highlights a prominent role of Gal-3 in the prognosis of HF patients, but a conflicting role in terms of prediction or early diagnosis of HF.
Repeated Measurements of Gal-3 Levels
While a single measurement of Gal-3 levels is informative, the main characteristic that justifies the use of Gal-3 for HF management is the lower biological variability compared to other cardiac markers, with the within-subject variability being equal to 8.1% in both healthy subjects and HF patients [136][124].
Since the low within-subject variability justifies the usefulness of repeated measurements, Gal-3 levels have been evaluated in patients with HF enrolled in the CORONA (n = 1329) and COACH (n = 324) trials, with repeated measurements at baseline and at 3 or 6 months, respectively. An increase of Gal-3 by >15% indicated a 50% higher relative hazard of adverse events (hospitalization and mortality) also after correction for age, sex, diabetes mellitus, left ventricular ejection fraction, renal function, medication, suggesting a significant prognostic value of repeated Gal-3 measurements [137][125]. As to the general population, in the longitudinal study carried out on 2477 participants in the Framingham Heart Study Offspring cohort, increases in Gal-3 levels during the observation time were related to future HF, CVD and all-cause mortality [138][126].
4. Gal-3 and Statins
In recent years, several studies have been carried out to investigate the regulation of Gal-3 levels by statin therapy, as well as the association of Gal-3 serum levels with the rate of cardiovascular events after statin therapy.
In a model of 36-week-old Apoe−/− mice fed a high-cholesterol diet for 5 days, the intraplaque Gal-3 levels were correlated with plaque inflammation. In this mouse model, the statin treatment markedly reduced both intraplaque Gal-3 levels and macrophage content compared to their saline-treated counterparts [54][36]. This study suggested the possibility of using Gal-3 as an indicator of plaque stabilization with statin therapy.
A recent study demonstrated an association of Gal-3-negative macrophages accumulation with atherosclerotic plaque development in Apoe−/− mice. This study also revealed that statin therapy induced plaque regression together with decreased accumulation of Gal-3-negative macrophages. These findings suggest that statins act by stimulating the protective role of Gal-3 in regulating macrophage polarization and in delaying plaque progression [59][41].
As for humans, the impact of statin therapy on Gal-3 levels in 78 consecutive patients (40 symptomatic, 38 asymptomatic) undergoing carotid endarterectomy has been investigated [52][34]. The authors showed an association between long-term statin treatment and elevated Gal-3 levels and reduced macrophage intra-plaque concentrations (as an indicator of plaque stability) compared to patients with short-term treatment. The study supports the hypothesis that long-term statin treatment induces an increase in the intra-plaque Gal-3 concentration, mediating plaque stabilization.
Although these two studies have been performed in mice and humans, respectively, they suggest two opposite regulations of Gal-3 levels by statins. Additional studies are required to disclose the real Gal-3 regulation.
An interesting study [127] [139] was conducted on 1492 patients with ischaemic systolic HF enrolled in the CORONA study. These patients were randomly assigned to 10 mg/day of rosuvastatin or placebo, and Gal-3 was measured in the plasma. Patients with Gal-3 values < 19.0 ng/mL treated with rosuvastatin showed a decreased risk of the primary endpoint (i.e., cardiovascular death, myocardial infarction, or stroke), lower total mortality and lower rate of the combined endpoint of total mortality and hospitalization for worsening HF compared to patients with Gal-3 > 19.0 ng/mL.
A recent review discussed the evidence surrounding a possible use of Gal-3 for the prediction of statin-therapy outcomes and suggested that low Gal-3 levels could help to identify a subset of subjects with a more functional myocardium [140][128].
5. Gal-3 as a Therapeutic Target for CVD
Since the pathogenic role of Gal-3 in CVD has been established, several hypotheses on its therapeutic use have been put forward [14,141][129][130].
As an example, the inhibitor MCP, an oligosaccharide belonging to the pectin family and present in the peels of fruits and vegetables, was reported to inhibit Gal-3 by binding to its Carbohydrate Recognition Domain [142][131]. In rodents showing LV diastolic dysfunction treated with aldosterone and salt, Gal-3 inhibition through MCP was able to reduce aldosterone-induced cardiac and renal fibrosis and to improve cardio-renal dysfunction [143][132].
In a murine model of HF with cardiac hyperaldosteronism, the use of MCP prevented the development of myocardial fibrosis and the combination of this molecule and mineralocorticoid receptor antagonists resulted in enhanced effects on cardiac inflammation and fibrosis, by reducing the development of myocardial inflammation and fibrogenesis [144][133]. On the other hand, treatment with mineralocorticoid receptor antagonists showed a down-regulation of Gal-3 expression in an experimental model of LV systolic dysfunction after AMI and such regulation correlated with lower expression levels of fibrosis [145][134].
Moreover, MCP turned out to be effective for treating atherosclerosis in mice. In fact, in high-cholesterol diet fed ApoE−/− mice, a well-characterized murine model of atherosclerosis, Gal-3 deletion as well as MCP oral administration reduced plaque volume [55][37].
References
- Newlaczyl, A.U.; Yu, L.-G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Nuclear transport of galectin-3 and its therapeutic implications. Semin. Cancer Biol. 2014, 27, 30–38. [Google Scholar] [CrossRef]
- Mazurek, N.; Conklin, J.; Byrd, J.C.; Raz, A.; Bresalier, R.S. Phosphorylation of the β-Galactoside-Binding Protein Galectin-3 Modulates Binding to Its Ligands. J. Biol. Chem. 2000, 275, 36311–36315. [Google Scholar] [CrossRef]
- Sève, A.P.; Felin, M.; Doyennette-Moyne, M.-A.; Sahraoui, T.; Aubery, M.; Hubert, J. Evidence for a lactose-mediated association between two nuclear carbohydrate-binding proteins. Glycobiology 1993, 3, 23–30. [Google Scholar] [CrossRef]Brewer, C. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin–saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef]
- Goletz, S.; Hanisch, F.G.; Karsten, U. Novel αGalNAc containing glycans on cytokeratins are recognized in vitro by galectins with type II carbohydrate recognition domains. J. Cell Sci. 1997, 110, 1585–1596. [Google Scholar]Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276.Sato, S.; Hughes, R.C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [PubMed]
- Menon, R.P.; Strom, M.; Hughes, R. Interaction of a novel cysteine and histidine-rich cytoplasmic protein with galectin-3 in a carbohydrate-independent manner. FEBS Lett. 2000, 470, 227–231. [Google Scholar] [CrossRef]Koths, K.; Taylor, E.; Halenbeck, R.; Casipit, C.; Wang, A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J. Biol. Chem. 1993, 268, 14245–14249. [Google Scholar]
- Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of galectin-1 and Gal-3 with Gemin4 in complexes containing the SMN protein. Nucleic Acids Res. 2001, 27, 3595–3602. [Google Scholar] [CrossRef]Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking Human Lectin Galectin-3 Interacts with Sulfated Glycosaminoglycans and Chondroitin Sulfate Proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef]
- Liu, F.T.; Patterson, R.J.; Wang, J.L. Intracellular functions of galectins. Biochim. Biophys. Acta 2002, 1572, 263–273. [Google Scholar] [CrossRef]Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- World Health Organization. Global Atlas on Cardiovascular Disease Prevention and Control; Mendis, S., Puska, P., Norrving, B., Eds.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]Suthahar, N.; Meijers, W.C.; Silljé, H.H.; Ho, J.E.; Liu, F.-T.; De Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin–saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef]Subhash, V.V.; Ling, S.S.M.; Ho, B. Extracellular galectin-3 counteracts adhesion and exhibits chemoattraction in Helicobacter pylori-infected gastric cancer cells. Microbiology 2016, 162, 1360–1366. [Google Scholar] [CrossRef]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-Glycan Number and Degree of Branching Cooperate to Regulate Cell Proliferation and Differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef] [PubMed]Wright, R.D.; Souza, P.R.; Flak, M.B.; Thedchanamoorthy, P.; Norling, L.V.; Cooper, D. Galectin-3-null mice display defective neutrophil clearance during acute inflammation. J. Leukoc. Biol. 2017, 101, 717–726. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [PubMed]Karlsson, A.; Christenson, K.; Matlak, M.; Björstad, Å.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 2008, 19, 16–20. [Google Scholar] [CrossRef]
- Koths, K.; Taylor, E.; Halenbeck, R.; Casipit, C.; Wang, A. Cloning and characterization of a human Mac-2-binding protein, a new member of the superfamily defined by the macrophage scavenger receptor cysteine-rich domain. J. Biol. Chem. 1993, 268, 14245–14249. [Google Scholar]Bambouskova, M.; Polakovicova, I.; Halova, I.; Goel, G.; Dráberová, L.; Bugajev, V.; Doan, A.; Utekal, P.; Gardet, A.; Xavier, R.J.; et al. New Regulatory Roles of Galectin-3 in High-Affinity IgE Receptor Signaling. Mol. Cell. Biol. 2016, 36, 1366–1382. [Google Scholar] [CrossRef]
- Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking Human Lectin Galectin-3 Interacts with Sulfated Glycosaminoglycans and Chondroitin Sulfate Proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef]Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies Used by Bacteria to Grow in Macrophages. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]Torina, A.; Villari, S.; Blanda, V.; Vullo, S.; La Manna, M.P.; Azgomi, M.S.S.; Di Liberto, D.; De La Fuente, J.; Sireci, G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 2020, 21, 5437. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.; Ho, J.E.; Liu, F.-T.; De Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276.
- Subhash, V.V.; Ling, S.S.M.; Ho, B. Extracellular galectin-3 counteracts adhesion and exhibits chemoattraction in Helicobacter pylori-infected gastric cancer cells. Microbiology 2016, 162, 1360–1366. [Google Scholar] [CrossRef]Torina, A.; Blanda, V.; Villari, S.; Piazza, A.; La Russa, F.; Grippi, F.; La Manna, M.P.; Di Liberto, D.; De La Fuente, J.; Sireci, G. Immune Response to Tick-Borne Hemoparasites: Host Adaptive Immune Response Mechanisms as Potential Targets for Therapies and Vaccines. Int. J. Mol. Sci. 2020, 21, 8813. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.D.; Souza, P.R.; Flak, M.B.; Thedchanamoorthy, P.; Norling, L.V.; Cooper, D. Galectin-3-null mice display defective neutrophil clearance during acute inflammation. J. Leukoc. Biol. 2017, 101, 717–726. [Google Scholar] [CrossRef]Rabinovich, G.A.; Baum, L.G.; Tinari, N.; Paganelli, R.; Natoli, C.; Liu, F.-T.; Iacobelli, S. Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002, 23, 313–320. [Google Scholar] [CrossRef]
- Karlsson, A.; Christenson, K.; Matlak, M.; Björstad, Å.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 2008, 19, 16–20. [Google Scholar] [CrossRef]Joo, H.G.; Goedegebuure, P.S.; Sadanaga, N.; Nagoshi, M.; Von Bernstorff, W.; Eberlein, T.J. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J. Leukoc. Biol. 2001, 69, 555–564. [Google Scholar]
- Bambouskova, M.; Polakovicova, I.; Halova, I.; Goel, G.; Dráberová, L.; Bugajev, V.; Doan, A.; Utekal, P.; Gardet, A.; Xavier, R.J.; et al. New Regulatory Roles of Galectin-3 in High-Affinity IgE Receptor Signaling. Mol. Cell. Biol. 2016, 36, 1366–1382. [Google Scholar] [CrossRef]Fortunato, G.; Di Taranto, M.D.; Bracale, U.M.; Del Guercio, L.; Carbone, F.; Mazzaccara, C.; Morgante, A.; D’Armiento, F.P.; D’Armiento, M.; Porcellini, M.; et al. Decreased Paraoxonase-2 Expression in Human Carotids during the Progression of Atherosclerosis. Arter. Thromb. Vasc. Biol. 2008, 28, 594–600. [Google Scholar] [CrossRef]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies Used by Bacteria to Grow in Macrophages. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Torina, A.; Villari, S.; Blanda, V.; Vullo, S.; La Manna, M.P.; Azgomi, M.S.S.; Di Liberto, D.; De La Fuente, J.; Sireci, G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 2020, 21, 5437. [Google Scholar] [CrossRef] [PubMed]Fortunato, G.; Di Taranto, M.D. Polymorphisms and the expression of genes encoding enzymes involved in cardiovascular diseases. Clin. Chim. Acta 2007, 381, 21–25. [Google Scholar] [CrossRef]
- Torina, A.; Blanda, V.; Villari, S.; Piazza, A.; La Russa, F.; Grippi, F.; La Manna, M.P.; Di Liberto, D.; De La Fuente, J.; Sireci, G. Immune Response to Tick-Borne Hemoparasites: Host Adaptive Immune Response Mechanisms as Potential Targets for Therapies and Vaccines. Int. J. Mol. Sci. 2020, 21, 8813. [Google Scholar] [CrossRef] [PubMed]Di Taranto, M.D.; Morgante, A.; Bracale, U.M.; D’Armiento, F.P.; Porcellini, M.; Bracale, G.; Fortunato, G.; Salvatore, F. Altered expression of inflammation-related genes in human carotid atherosclerotic plaques. Atherosclerosis 2012, 220, 93–101. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Baum, L.G.; Tinari, N.; Paganelli, R.; Natoli, C.; Liu, F.-T.; Iacobelli, S. Galectins and their ligands: Amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002, 23, 313–320. [Google Scholar] [CrossRef]Poredoš, P.; Ježovnik, M.K. Markers of preclinical atherosclerosis and their clinical relevance. Vasa 2015, 44, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.G.; Goedegebuure, P.S.; Sadanaga, N.; Nagoshi, M.; Von Bernstorff, W.; Eberlein, T.J. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J. Leukoc. Biol. 2001, 69, 555–564. [Google Scholar]Willeit, P.; Thompson, S.G.; Agewall, S.; Bergström, G.; Bickel, H.; Catapano, A.L.; Chien, K.-L.; De Groot, E.; Empana, J.-P.; Etgen, T.; et al. Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur. J. Prev. Cardiol. 2016, 23, 194–205. [Google Scholar] [CrossRef]
- Fortunato, G.; Di Taranto, M.D.; Bracale, U.M.; Del Guercio, L.; Carbone, F.; Mazzaccara, C.; Morgante, A.; D’Armiento, F.P.; D’Armiento, M.; Porcellini, M.; et al. Decreased Paraoxonase-2 Expression in Human Carotids during the Progression of Atherosclerosis. Arter. Thromb. Vasc. Biol. 2008, 28, 594–600. [Google Scholar] [CrossRef]Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Hear. J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 5284728. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.; Di Taranto, M.D. Polymorphisms and the expression of genes encoding enzymes involved in cardiovascular diseases. Clin. Chim. Acta 2007, 381, 21–25. [Google Scholar] [CrossRef]Nachtigal, M.; Al-Assaad, Z.; Mayer, E.P.; Kim, K.; Monsigny, M. Galectin-3 expression in human atherosclerotic lesions. Am. J. Pathol. 1998, 152, 10. [Google Scholar]
- Di Taranto, M.D.; Morgante, A.; Bracale, U.M.; D’Armiento, F.P.; Porcellini, M.; Bracale, G.; Fortunato, G.; Salvatore, F. Altered expression of inflammation-related genes in human carotid atherosclerotic plaques. Atherosclerosis 2012, 220, 93–101. [Google Scholar] [CrossRef]Pugliese, G.; Iacobini, C.; Fantauzzi, C.B.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Poredoš, P.; Ježovnik, M.K. Markers of preclinical atherosclerosis and their clinical relevance. Vasa 2015, 44, 247–256. [Google Scholar] [CrossRef] [PubMed]Menini, S.; Iacobini, C.; Ricci, C.; Fantauzzi, C.B.; Salvi, L.; Pesce, C.M.; Relucenti, M.; Familiari, G.; Taurino, M.; Pugliese, G. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc. Res. 2013, 100, 472–480. [Google Scholar] [CrossRef]
- Willeit, P.; Thompson, S.G.; Agewall, S.; Bergström, G.; Bickel, H.; Catapano, A.L.; Chien, K.-L.; De Groot, E.; Empana, J.-P.; Etgen, T.; et al. Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur. J. Prev. Cardiol. 2016, 23, 194–205. [Google Scholar] [CrossRef]Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arter. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Soeki, T.; Sata, M. Inflammatory Biomarkers and Atherosclerosis. Int. Hear. J. 2016, 57, 134–139. [Google Scholar] [CrossRef] [PubMed]Djordjevic, A.; Zivkovic, M.; Stankovic, A.; Zivotic, I.; Koncar, I.; Davidovic, L.; Alavantic, D.; Djurić, T. Genetic Variants in the Vicinity ofLGALS-3Gene andLGALS-3 mRNA Expression in Advanced Carotid Atherosclerosis: An Exploratory Study. J. Clin. Lab. Anal. 2016, 30, 1150–1157. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Z.; Wang, R.; Zheng, Y.; Li, H.; Yang, L. Galectin-3 Is a Potential Mediator for Atherosclerosis. J. Immunol. Res. 2020, 2020, 5284728. [Google Scholar] [CrossRef] [PubMed]Kadoglou, N.; Sfyroeras, G.; Spathis, A.; Gkekas, C.; Gastounioti, A.; Mantas, G.; Nikita, K.; Karakitsos, P.; Liapis, C.D. Galectin-3, Carotid Plaque Vulnerability, and Potential Effects of Statin Therapy. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 4–9. [Google Scholar] [CrossRef]
- Nachtigal, M.; Al-Assaad, Z.; Mayer, E.P.; Kim, K.; Monsigny, M. Galectin-3 expression in human atherosclerotic lesions. Am. J. Pathol. 1998, 152, 10. [Google Scholar]Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V.; Cordone, S.; Taurino, M.; Serino, M.; Marano, G.; Federici, M.; et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: Role of lipoxidation via receptor-mediated mechanisms. Arter. Thromb. Vasc. Biol. 2009, 29, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Iacobini, C.; Fantauzzi, C.B.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]Lee, Y.-J.; Koh, Y.-S.; Park, H.E.; Lee, H.J.; Hwang, B.-H.; Kang, M.-K.; Lee, S.-Y.; Kim, P.-J.; Ihm, S.H.; Seung, K.-B.; et al. Spatial and Temporal Expression, and Statin Responsiveness of Galectin-1 and Galectin-3 in Murine Atherosclerosis. Korean Circ. J. 2013, 43, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Fantauzzi, C.B.; Salvi, L.; Pesce, C.M.; Relucenti, M.; Familiari, G.; Taurino, M.; Pugliese, G. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc. Res. 2013, 100, 472–480. [Google Scholar] [CrossRef]Lu, Y.; Zhang, M.; Zhao, P.; Jia, M.; Liu, B.; Jia, Q.; Guo, J.; Dou, L.; Li, J. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol. Med. Rep. 2017, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arter. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef]Nachtigal, M.; Ghaffar, A.; Mayer, E.P. Galectin-3 Gene Inactivation Reduces Atherosclerotic Lesions and Adventitial Inflammation in ApoE-Deficient Mice. Am. J. Pathol. 2008, 172, 247–255. [Google Scholar] [CrossRef]
- Djordjevic, A.; Zivkovic, M.; Stankovic, A.; Zivotic, I.; Koncar, I.; Davidovic, L.; Alavantic, D.; Djurić, T. Genetic Variants in the Vicinity ofLGALS-3Gene andLGALS-3 mRNA Expression in Advanced Carotid Atherosclerosis: An Exploratory Study. J. Clin. Lab. Anal. 2016, 30, 1150–1157. [Google Scholar] [CrossRef]MacKinnon, A.C.; Liu, X.; Hadoke, P.W.; Miller, M.R.; Newby, D.E.; Sethi, T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 2013, 23, 654–663. [Google Scholar] [CrossRef]
- Kadoglou, N.; Sfyroeras, G.; Spathis, A.; Gkekas, C.; Gastounioti, A.; Mantas, G.; Nikita, K.; Karakitsos, P.; Liapis, C.D. Galectin-3, Carotid Plaque Vulnerability, and Potential Effects of Statin Therapy. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 4–9. [Google Scholar] [CrossRef]Sun, Z.; Wang, Z.; Li, L.; Yan, J.; Shao, C.; Bao, Z.; Jing, L.; Pang, Q.; Geng, Y.; Zhang, L. RAGE/galectin-3 yields intraplaque calcification transformation via sortilin. Acta Diabetol. 2019, 56, 457–472. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V.; Cordone, S.; Taurino, M.; Serino, M.; Marano, G.; Federici, M.; et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: Role of lipoxidation via receptor-mediated mechanisms. Arter. Thromb. Vasc. Biol. 2009, 29, 831–836. [Google Scholar] [CrossRef] [PubMed]Di Gregoli, K.; Somerville, M.; Bianco, R.; Thomas, A.C.; Frankow, A.; Newby, A.C.; George, S.J.; Jackson, C.L.; Johnson, J.L. Galectin-3 Identifies a Subset of Macrophages with a Potential Beneficial Role in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 1491–1509. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Koh, Y.-S.; Park, H.E.; Lee, H.J.; Hwang, B.-H.; Kang, M.-K.; Lee, S.-Y.; Kim, P.-J.; Ihm, S.H.; Seung, K.-B.; et al. Spatial and Temporal Expression, and Statin Responsiveness of Galectin-1 and Galectin-3 in Murine Atherosclerosis. Korean Circ. J. 2013, 43, 223–230. [Google Scholar] [CrossRef] [PubMed]Li, L.-C.; Li, J.; Gao, J. Functions of Galectin-3 and Its Role in Fibrotic Diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Zhao, P.; Jia, M.; Liu, B.; Jia, Q.; Guo, J.; Dou, L.; Li, J. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol. Med. Rep. 2017, 16, 647–653. [Google Scholar] [CrossRef] [PubMed]Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. CAMP and Epac in the Regulation of Tissue Fibrosis: CAMP and Fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef]
- Nachtigal, M.; Ghaffar, A.; Mayer, E.P. Galectin-3 Gene Inactivation Reduces Atherosclerotic Lesions and Adventitial Inflammation in ApoE-Deficient Mice. Am. J. Pathol. 2008, 172, 247–255. [Google Scholar] [CrossRef]Speca, S. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Liu, X.; Hadoke, P.W.; Miller, M.R.; Newby, D.E.; Sethi, T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 2013, 23, 654–663. [Google Scholar] [CrossRef]Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; Van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Z.; Li, L.; Yan, J.; Shao, C.; Bao, Z.; Jing, L.; Pang, Q.; Geng, Y.; Zhang, L. RAGE/galectin-3 yields intraplaque calcification transformation via sortilin. Acta Diabetol. 2019, 56, 457–472. [Google Scholar] [CrossRef]Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; Van Goor, H.; Sanjabi, B.; Van Der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Hear. Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Di Gregoli, K.; Somerville, M.; Bianco, R.; Thomas, A.C.; Frankow, A.; Newby, A.C.; George, S.J.; Jackson, C.L.; Johnson, J.L. Galectin-3 Identifies a Subset of Macrophages with a Potential Beneficial Role in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 1491–1509. [Google Scholar] [CrossRef]Sato, S.; Hughes, R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar]
- Li, L.-C.; Li, J.; Gao, J. Functions of Galectin-3 and Its Role in Fibrotic Diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef]Kasper, M.; Hughes, R.C. Immunocytochemical Evidence for a Modulation of Galectin 3 (Mac-2), a Carbohydrate Binding Protein, in Pulmonary Fibrosis. J. Pathol. 1996, 179, 309–316. [Google Scholar] [CrossRef]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. CAMP and Epac in the Regulation of Tissue Fibrosis: CAMP and Fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef]
- Speca, S. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; Van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; Van Goor, H.; Sanjabi, B.; Van Der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Hear. Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar]
- Kasper, M.; Hughes, R.C. Immunocytochemical Evidence for a Modulation of Galectin 3 (Mac-2), a Carbohydrate Binding Protein, in Pulmonary Fibrosis. J. Pathol. 1996, 179, 309–316. [Google Scholar] [CrossRef]
- Insel, P.A.; Murray, F.; Yokoyama, U.; Romano, S.; Yun, H.; Brown, L.; Snead, A.; Lu, D.; Aroonsakool, N. CAMP and Epac in the Regulation of Tissue Fibrosis: CAMP and Fibrosis. Br. J. Pharmacol. 2012, 166, 447–456. [Google Scholar] [CrossRef]Sanchez-Mas, J.; Lax, A.; Asensio-Lopez, M.; Palacio, M.F.-D.; Caballero, L.; Garrido, I.; Pastor, F.; Januzzi, J.; Pascual-Figal, D.A. Galectin-3 expression in cardiac remodeling after myocardial infarction. Int. J. Cardiol. 2014, 172, e98–e101. [Google Scholar] [CrossRef]
- Speca, S. Cellular and molecular mechanisms of intestinal fibrosis. World J. Gastroenterol. 2012, 18, 3635–3661. [Google Scholar] [CrossRef]Liu, Y.-H.; D’Ambrosio, M.; Liao, T.-D.; Peng, H.; Rhaleb, N.-E.; Sharma, U.; André, S.; Gabius, H.-J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; Van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]Garner, O.B.; Baum, L.G. Galectin–glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem. Soc. Trans. 2008, 36, 1472–1477. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; Van Goor, H.; Sanjabi, B.; Van Der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Hear. Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar]Suthahar, N.; Meijers, W.C.; Silljé, H.H.; De Boer, R.A. From Inflammation to Fibrosis—Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Hear. Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef]
- Kasper, M.; Hughes, R.C. Immunocytochemical Evidence for a Modulation of Galectin 3 (Mac-2), a Carbohydrate Binding Protein, in Pulmonary Fibrosis. J. Pathol. 1996, 179, 309–316. [Google Scholar] [CrossRef]Ibarrola, J.; Matilla, L.; Martínez-Martínez, E.; Gueret, A.; Fernández-Celis, A.; Henry, J.-P.; Nicol, L.; Jaisser, F.; Mulder, P.; Ouvrard-Pascaud, A.; et al. Myocardial Injury after Ischemia/Reperfusion Is Attenuated by Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019, 9, 9607. [Google Scholar] [CrossRef]
- Sanchez-Mas, J.; Lax, A.; Asensio-Lopez, M.; Palacio, M.F.-D.; Caballero, L.; Garrido, I.; Pastor, F.; Januzzi, J.; Pascual-Figal, D.A. Galectin-3 expression in cardiac remodeling after myocardial infarction. Int. J. Cardiol. 2014, 172, e98–e101. [Google Scholar] [CrossRef]Zhang, M.; Cheng, K.; Chen, H.; Tu, J.; Shen, Y.; Pang, L.; Wu, W. Galectin-3 knock down inhibits cardiac ischemia-reperfusion injury through interacting with bcl-2 and modulating cell apoptosis. Arch. Biochem. Biophys. 2020, 694, 108602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; D’Ambrosio, M.; Liao, T.-D.; Peng, H.; Rhaleb, N.-E.; Sharma, U.; André, S.; Gabius, H.-J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef]Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.C.; Pokharel, S.; Van Brakel, T.J.; Van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.-J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ruifrok, W.P.T.; Meissner, M.; Bos, E.M.; Van Goor, H.; Sanjabi, B.; Van Der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering With Myocardial Fibrogenesis. Circ. Hear. Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef]Screever, E.M.; Meijers, W.C.; Van Veldhuisen, D.J.; De Boer, R.A. New developments in the pharmacotherapeutic management of heart failure in elderly patients: Concerns and considerations. Expert Opin. Pharmacother. 2017, 18, 645–655. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar]Gehlken, C.; Suthahar, N.; Meijers, W.C.; De Boer, R.A. Galectin-3 in Heart Failure. Hear. Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef]
- Kasper, M.; Hughes, R.C. Immunocytochemical Evidence for a Modulation of Galectin 3 (Mac-2), a Carbohydrate Binding Protein, in Pulmonary Fibrosis. J. Pathol. 1996, 179, 309–316. [Google Scholar] [CrossRef]De Boer, R.A.; Voors, A.A.; Muntendam, P.; Van Gilst, W.H.; Van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Hear. Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- Sanchez-Mas, J.; Lax, A.; Asensio-Lopez, M.; Palacio, M.F.-D.; Caballero, L.; Garrido, I.; Pastor, F.; Januzzi, J.; Pascual-Figal, D.A. Galectin-3 expression in cardiac remodeling after myocardial infarction. Int. J. Cardiol. 2014, 172, e98–e101. [Google Scholar] [CrossRef]Clementy, N.; Garcia, B.; André, C.; Bisson, A.; Benhenda, N.; Pierre, B.; Bernard, A.; Fauchier, L.; Piver, E.; Babuty, M. Galectin-3 level predicts response to ablation and outcomes in patients with persistent atrial fibrillation and systolic heart failure. PLoS ONE 2018, 13, e0201517. [Google Scholar] [CrossRef]
- Liu, Y.-H.; D’Ambrosio, M.; Liao, T.-D.; Peng, H.; Rhaleb, N.-E.; Sharma, U.; André, S.; Gabius, H.-J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef]Kim, H.; Lee, J.; Hyun, J.W.; Park, J.W.; Joo, H.; Shin, T. Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol. Int. 2007, 31, 655–662. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Liu, Y.; Zhang, M.; Wang, Y.; Hu, L. Ticagrelor Attenuates Myocardial Ischaemia-Reperfusion Injury Possibly through Downregulating Galectin-3 Expression in the Infarct Area of Rats: Ticagrelor Protects Ischaemic Heart and Downregulates Galectin-3 Expression. Br. J. Clin. Pharmacol. 2018, 84, 1180–1186. [Google Scholar] [CrossRef]
- Nguyen, M.-N.; Su, Y.; Vizi, D.; Fang, L.; Ellims, A.H.; Zhao, W.-B.; Kiriazis, H.; Gao, X.-M.; Sadoshima, J.; Taylor, A.J.; et al. Mechanisms responsible for increased circulating levels of galectin-3 in cardiomyopathy and heart failure. Sci. Rep. 2018, 8, 8213. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Arora, R.; Green, D.; Greenland, P.; Lee, D.C.; Lloyd-Jones, D.M.; Markl, M.; Ng, J.; Shah, S.J. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation 2015, 132, 278–291. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of Transforming Growth Factor-β1–driven Lung Fibrosis by Galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Inácio, A.R.; Ruscher, K.; Leng, L.; Bucala, R.; Deierborg, T. Macrophage Migration Inhibitory Factor Promotes Cell Death and Aggravates Neurologic Deficits after Experimental Stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1093–1106. [Google Scholar] [CrossRef]
- DeRoo, E.P.; Wrobleski, S.K.; Shea, E.M.; Al-Khalil, R.K.; Hawley, A.E.; Henke, P.K.; Myers, D.D., Jr.; Wakefield, T.W.; Diaz, J.A. The role of galectin-3 and galectin-3–binding protein in venous thrombosis. Blood 2015, 125, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, M.M.; Pejnovic, N.; Mitrovic, S.; Jovicic, N.; Petrovic, I.; Arsenijevic, N.; Lukic, M.L.; Ljujic, B. Galectin-3 deficiency enhances type 2 immune cell-mediated myocarditis in mice. Immunol. Res. 2018, 66, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, N.; Gulizia, M.M.; Clerico, A.; Di Tano, G.; Emdin, M.; Feola, M.; Iacoviello, M.; Latini, R.; Mortara, A.; Valle, R.; et al. ANMCO/ELAS/SIBioC Consensus Document: Biomarkers in heart failure. Eur. Hear. J. Suppl. 2017, 19 (Suppl. D), D102–D112. [Google Scholar] [CrossRef] [PubMed]
- Acampa, W.; Di Taranto, M.D.; Morgante, A.; Salvatore, B.; Evangelista, L.; Ricci, F.; Costanzo, P.; De Sisto, E.; Filardi, P.P.; Petretta, M.; et al. C-reactive protein levels are associated with paraoxonase polymorphism L55M in patients undergoing cardiac SPECT imaging. Scand. J. Clin. Lab. Investig. 2011, 71, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Di Taranto, M.D.; D’Agostino, M.N.; Marotta, G.; Gentile, M.; Abate, G.; Mirabelli, P.; Di Noto, R.; Del Vecchio, L.; Rubba, P.; et al. Identification and functional characterization of LDLR mutations in familial hypercholesterolemia patients from Southern Italy. Atherosclerosis 2010, 210, 493–496. [Google Scholar] [CrossRef]
- Viereck, J.; Thum, T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017, 120, 381–399. [Google Scholar] [CrossRef]
- Averna, M.; Cefalù, A.B.; Casula, M.; Noto, D.; Arca, M.; Bertolini, S.; Calandra, S.; Catapano, A.L.; Tarugi, P.; Pellegatta, F.; et al. Familial Hypercholesterolemia: The Italian Atherosclerosis Society Network (LIPIGEN). Atheroscler. Suppl. 2017, 29, 11–16. [Google Scholar] [CrossRef]
- Bei, Y.; Yu, P.; Cretoiu, D.; Cretoiu, S.M.; Xiao, J. Exosomes-Based Biomarkers for the Prognosis of Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 998, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, M.D.; Staiano, A.; D’Agostino, M.N.; D’Angelo, A.; Bloise, E.; Morgante, A.; Marotta, G.; Gentile, M.; Rubba, P.; Fortunato, G. Association of USF1 and APOA5 polymorphisms with familial combined hyperlipidemia in an Italian population. Mol. Cell. Probes 2015, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Kouvelos, G.; Paolocci, N. Circulating biomarkers for cardiovascular diseases: The beats never stop. Acta Pharmacol. Sin. 2018, 39, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Bellia, C.; Sasso, B.L.; Pivetti, A.; Muratore, M.; Scazzone, C.; Bivona, G.; Lippi, G.; Ciaccio, M. Establishing the upper reference limit of Galectin-3 in healthy blood donors. Biochem. Med. 2017, 27, 030709. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef] [PubMed]
- Lyngbakken, M.N.; Myhre, P.L.; Røsjø, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef]
- Falcone, C.; Lucibello, S.; Mazzucchelli, I.; Bozzini, S.; D’Angelo, A.; Schirinzi, S.; Totaro, R.; Falcone, R.; Bondesan, M.; Pelissero, G. Galectin-3 Plasma Levels and Coronary Artery Disease: A New Possible Biomarker of Acute Coronary Syndrome. Int. J. Immunopathol. Pharmacol. 2011, 24, 905–913. [Google Scholar] [CrossRef]
- Rubba, P.; Gentile, M.; Marotta, G.; Iannuzzi, A.; Sodano, M.; De Simone, B.; Jossa, F.; Iannuzzo, G.; Giacobbe, C.; Di Taranto, M.D.; et al. Causative mutations and premature cardiovascular disease in patients with heterozygous familial hypercholesterolaemia. Eur. J. Prev. Cardiol. 2017, 24, 1051–1059. [Google Scholar] [CrossRef]
- Di Taranto, M.D.; Giacobbe, C.; Buonaiuto, A.; Calcaterra, I.; Palma, D.; Maione, G.; Iannuzzo, G.; Di Minno, M.N.D.; Rubba, P.; Fortunato, G. A Real-World Experience of Clinical, Biochemical and Genetic Assessment of Patients with Homozygous Familial Hypercholesterolemia. J. Clin. Med. 2020, 9, 219. [Google Scholar] [CrossRef]
- Eliaz, I.; Weil, E.; Dutton, J.-A.; McCalley, A.E.; Nolte, B.; Moriarty, P.M. Lipoprotein apheresis reduces circulating galectin-3 in humans. J. Clin. Apher. 2016, 31, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Oyenuga, A.; Folsom, A.R.; Fashanu, O.; Aguilar, D.; Ballantyne, C.M. Plasma Galectin-3 and Sonographic Measures of Carotid Atherosclerosis in the Atherosclerosis Risk in Communities Study. Angiology 2019, 70, 47–55. [Google Scholar] [CrossRef]
- Ciaccio, M.; Agnello, L.; Bracale, U.M.; Di Taranto, M.D.; Ciaccio, M.; Bracale, U.M.; Giacobbe, C.; Fortunato, G.; Travaglino, A.; Palma, D.; et al. Galectin-3 and Lp(a) plasma concentrations and advanced carotid atherosclerotic plaques: Correlation with plaque presence and features. Biochim. Clin. 2019, 43, 289–295. [Google Scholar] [CrossRef]
- Lisowska, A.; Knapp, M.; Tycińska, A.; Motybel, E.; Kamiński, K.; Święcki, P.; Musiał, W.J.; Dymicka-Piekarska, V. Predictive value of Galectin-3 for the occurrence of coronary artery disease and prognosis after myocardial infarction and its association with carotid IMT values in these patients: A mid-term prospective cohort study. Atherosclerosis 2016, 246, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.B.; Fuster, V.; Muntendam, P.; Mehran, R.; Baber, U.; Sartori, S.; Falk, E. Negative Risk Markers for Cardiovascular Events in the Elderly. J. Am. Coll. Cardiol. 2019, 74, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Pedon, L.; Cesari, M.; Frigo, A.C.; Azzolini, M.; Plebani, M.; Rossi, G.P. Galectin-3 Predicts Long-Term Cardiovascular Death in High-Risk Patients with Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2015, 35, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Aksan, G.; Gedikli, Ö.; Keskin, K.; Nar, G.; Inci, S.; Yıldız, S.S.; Kaplan, Ö.; Soylu, K.; Kılıçkesmez, K.O.; Şahin, M.; et al. Is galectin-3 a biomarker, a player—or both—in the presence of coronary atherosclerosis? J. Investig. Med. 2016, 64, 764–770. [Google Scholar] [CrossRef]
- Święcki, P.; Sawicki, R.; Knapp, M.; Kamiński, K.A.; Ptaszyńska-Kopczyńska, K.; Sobkowicz, B.; Lisowska, A. Galectin-3 as the Prognostic Factor of Adverse Cardiovascular Events in Long-Term Follow up in Patients after Myocardial Infarction—A Pilot Study. J. Clin. Med. 2020, 9, 1640. [Google Scholar] [CrossRef]
- Asleh, R.; Enriquez-Sarano, M.; Jaffe, A.S.; Manemann, S.M.; Weston, S.A.; Jiang, R.; Roger, V.L. Galectin-3 Levels and Outcomes after Myocardial Infarction: A Population-Based Study. J. Am. Coll. Cardiol. 2019, 73, 2286–2295. [Google Scholar] [CrossRef]
- Huttin, O.; Mandry, D.; Popovic, B.; Rossignol, P.; Odille, F.; Micard, E.; Lamiral, Z.; Zannad, F.; Girerd, N.; Marie, P.-Y. Plasma Galectin-3 predicts deleterious vascular dysfunction affecting post-myocardial infarction patients: An explanatory study. PLoS ONE 2020, 15, e0232572. [Google Scholar] [CrossRef]
- Lorenzo-Almorós, A.; Pello, A.; Aceña, Á.; Martínez-Milla, J.; Lorenzo, Ó.; Tarín, N.; Cristóbal, C.; Blanco-Colio, L.M.; Martín-Ventura, J.-L.; Huelmos, A.; et al. Galectin-3 is Associated with Cardiovascular Events in Post-Acute Coronary Syndrome Patients with Type-2 Diabetes. J. Clin. Med. 2020, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Ma, X.; Yu, H.; Chen, X. The predictive value of serum galectin 3 for abdominal aortic calcification in maintenance hemodialysis patients: A prospective cohort study. Hemodial. Int. 2020, 24, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, Z.-H.; Zhang, N.; Liu, S.-D.; Zhao, J.-J.; Liu, S.-Y. Serum Galectin-3 level, not Galectin-1, is associated with the clinical feature and outcome in patients with acute ischemic stroke. Oncotarget 2017, 8, 109752–109761. [Google Scholar] [CrossRef] [PubMed]
- He, X.-W.; Li, W.-L.; Li, C.; Liu, P.; Shen, Y.-G.; Zhu, M.; Jin, X.-P. Serum levels of galectin-1, galectin-3, and galectin-9 are associated with large artery atherosclerotic stroke. Sci. Rep. 2017, 7, 40994. [Google Scholar] [CrossRef]
- Edsfeldt, A.; Bengtsson, E.; Fredrikson, G.N.; Asciutto, G.; Dunér, P.; Björkbacka, H.; Nilsson, J.-Å.; Goncalves, I. High Plasma Levels of Galectin-3 Are Associated with Increased Risk for Stroke after Carotid Endarterectomy. Cerebrovasc. Dis. 2016, 41, 199–203. [Google Scholar] [CrossRef]
- Fort-Gallifa, I.; Hernández-Aguilera, A.; García-Heredia, A.; Cabré, N.; Luciano-Mateo, F.; Simó, J.M.; Martín-Paredero, V.; Camps, J.; Joven, J. Galectin-3 in Peripheral Artery Disease. Relationships with Markers of Oxidative Stress and Inflammation. IJMS 2017, 18, 973. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Lindholt, J.S.; Fernandez-Garcia, C.E.; Benito-Martin, A.; Burillo, E.; Zalba, G.; Beloqui, O.; Llamas-Granda, P.; Ortiz, A.; Egido, J.; et al. Galectin-3, a Biomarker Linking Oxidative Stress and Inflammation with the Clinical Outcomes of Patients with Atherothrombosis. J. Am. Hear. Assoc. 2014, 3, e000785. [Google Scholar] [CrossRef]
- Ding, N.; Yang, C.; Ballew, S.H.; Kalbaugh, C.A.; McEvoy, J.W.; Salameh, M.; Aguilar, D.; Hoogeveen, R.C.; Nambi, V.; Selvin, E.; et al. Fibrosis and Inflammatory Markers and Long-Term Risk of Peripheral Artery Disease: The ARIC Study. Arter. Thromb. Vasc. Biol. 2020, 40, 2322–2331. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Amin, H.Z.; Amin, L.Z.; Wijaya, I.P. GALECTIN-3: A Novel Biomarker for the Prognosis of Heart Failure. Clujul. Med. 2017, 90, 129–132. [Google Scholar] [CrossRef]
- McCullough, P.A.; Olobatoke, A.; Vanhecke, T.E. Galectin-3: A novel blood test for the evaluation and management of patients with heart failure. Rev. Cardiovasc. Med. 2011, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Januzzi, J.L.; Defilippi, C.; Adourian, A.S.; Shah, S.J.; Van Veldhuisen, D.J.; De Boer, R.A. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Hear. J. 2014, 167, 853–860.e4. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Adourian, A.; Muntendam, P.; Cohn, J.N. Baseline and serial measurements of galectin-3 in patients with heart failure: Relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur. J. Hear. Fail. 2013, 15, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Shin, H.J.; Mathenge, N.; Wang, F.; Kim, B.; Joseph, J.; Gaziano, J.M.; Djoussé, L. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients with Heart Failure and in the General Population. Am. J. Cardiol. 2017, 119, 57–64. [Google Scholar] [CrossRef]
- Demissei, B.G.; Cotter, G.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.; Pang, P.S.; Ponikowski, P.; Severin, T.M.; Wang, Y.; et al. A Multimarker Multi-Time Point-Based Risk Stratification Strategy in Acute Heart Failure: Results from the RELAX-AHF Trial: A Multimarker Multi-Time Point-Based Risk Stratification Strategy in AHF. Eur. J. Hear. Fail. 2017, 19, 1001–1010. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Fang, J.; Wang, R.; Nie, W. Circulating galectin-3 on admission and prognosis in acute heart failure patients: A meta-analysis. Hear. Fail. Rev. 2020, 25, 331–341. [Google Scholar] [CrossRef]
- Felker, G.M.; Fiuzat, M.; Shaw, L.K.; Clare, R.; Whellan, D.J.; Bettari, L.; Shirolkar, S.C.; Donahue, M.; Kitzman, D.W.; Zannad, F.; et al. Galectin-3 in ambulatory patients with heart failure: Results from the HF-ACTION study. Circ. Hear. Fail. 2012, 5, 72–78. [Google Scholar] [CrossRef]
- Lok, D.J.A.; Van Der Meer, P.; De La Porte, P.W.B.-A.; Lipsic, E.; Van Wijngaarden, J.; Hillege, H.L.; Van Veldhuisen, D.J. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: Data from the DEAL-HF study. Clin. Res. Cardiol. 2010, 99, 323–328. [Google Scholar] [CrossRef]
- Han, X.; Zhang, S.; Chen, Z.; Adhikari, B.K.; Zhang, Y.; Zhang, J.; Sun, J.; Wang, Y. Cardiac biomarkers of heart failure in chronic kidney disease. Clin. Chim. Acta 2020, 510, 298–310. [Google Scholar] [CrossRef]
- Tummalapalli, S.L.; Zelnick, L.R.; Andersen, A.H.; Christenson, R.H.; Defilippi, C.R.; Deo, R.; Go, A.S.; He, J.; Ky, B.; Lash, J.P.; et al. Association of Cardiac Biomarkers with the Kansas City Cardiomyopathy Questionnaire in Patients with Chronic Kidney Disease without Heart Failure. J. Am. Hear. Assoc. 2020, 9, e014385. [Google Scholar] [CrossRef]
- Suthahar, N.; Lau, E.S.; Blaha, M.J.; Paniagua, S.M.; Larson, M.G.; Psaty, B.M.; Benjamin, E.J.; Allison, M.A.; Bartz, T.M.; Januzzi, J.L.; et al. Sex-Specific Associations of Cardiovascular Risk Factors and Biomarkers with Incident Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1455–1465. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Seliger, S.; Defilippi, C.R.; Christenson, R.; Baldridge, A.S.; Kizer, J.R.; Psaty, B.M.; Shah, S.J. Relation of Biomarkers of Cardiac Injury, Stress, and Fibrosis with Cardiac Mechanics in Patients ≥ 65 Years of Age. Am. J. Cardiol. 2020, 136, 156–163. [Google Scholar] [CrossRef]
- Aguilar, D.; Sun, C.; Hoogeveen, R.C.; Nambi, V.; Selvin, E.; Matsushita, K.; Saeed, A.; McEvoy, J.W.; Shah, A.M.; Solomon, S.D.; et al. Levels and Change in Galectin-3 and Association with Cardiovascular Events: The ARIC Study. J. Am. Hear. Assoc. 2020, 9, e015405. [Google Scholar] [CrossRef]
- Meijers, W.C.; Van Der Velde, A.R.; Kobold, A.C.M.; Dijck-Brouwer, J.; Wu, A.H.; Jaffe, A.S.; De Boer, R.A. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur. J. Hear. Fail. 2017, 19, 357–365. [Google Scholar] [CrossRef]
- Van Der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; Van Veldhuisen, D.J.; De Boer, R.A. Prognostic Value of Changes in Galectin-3 Levels over Time in Patients with Heart Failure: Data from CORONA and COACH. Circ. Hear. Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; De Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.H.; Hulthe, J.; Muntendam, P.; Adourian, A.; Böhm, M.; Van Veldhuisen, D.J.; Komajda, M.; et al. Galectin-3 predicts response to statin therapy in the controlled rosuvastatin multinational trial in heart failure (CORONA). Eur. Hear. J. 2012, 33, 2290–2296. [Google Scholar] [CrossRef]
- Sharim, J.; Daniels, L.B. Soluble ST2 and Soluble Markers of Fibrosis: Emerging Roles for Prognosis and Guiding Therapy. Curr. Cardiol. Rep. 2020, 22, 41. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Bivona, G.; Sasso, B.L.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The Impact of Galectin-3 Inhibition on Aldosterone-Induced Cardiac and Renal Injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef]
- Vergaro, G.; Prud’Homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.-L.; Cohen-Solal, A.; Delcayre, C. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension 2016, 67, 606–612. [Google Scholar] [CrossRef]
- Lax, A.; Sanchez-Mas, J.; Asensio-Lopez, M.C.; Palacio, M.J.F.-D.; Caballero, L.; Garrido, I.P.; Pastor-Pérez, F.J.; Januzzi, J.L.; Pascual-Figal, D.A. Mineralocorticoid Receptor Antagonists Modulate Galectin-3 and Interleukin-33/ST2 Signaling in Left Ventricular Systolic Dysfunction after Acute Myocardial Infarction. JACC Heart Fail. 2015, 3, 50–58. [Google Scholar] [CrossRef]
- Aguilar, D.; Sun, C.; Hoogeveen, R.C.; Nambi, V.; Selvin, E.; Matsushita, K.; Saeed, A.; McEvoy, J.W.; Shah, A.M.; Solomon, S.D.; et al. Levels and Change in Galectin-3 and Association with Cardiovascular Events: The ARIC Study. J. Am. Hear. Assoc. 2020, 9, e015405. [Google Scholar] [CrossRef]
- Meijers, W.C.; Van Der Velde, A.R.; Kobold, A.C.M.; Dijck-Brouwer, J.; Wu, A.H.; Jaffe, A.S.; De Boer, R.A. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur. J. Hear. Fail. 2017, 19, 357–365. [Google Scholar] [CrossRef]
- Van Der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; Van Veldhuisen, D.J.; De Boer, R.A. Prognostic Value of Changes in Galectin-3 Levels over Time in Patients with Heart Failure: Data from CORONA and COACH. Circ. Hear. Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Ghorbani, A.; Bhambhani, V.; Christenson, R.H.; Meijers, W.C.; De Boer, R.A.; Levy, D.; Larson, M.G.; Ho, J.E. Longitudinal Change in Galectin-3 and Incident Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2018, 72, 3246–3254. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Kjekshus, J.; Nymo, S.H.; Hulthe, J.; Muntendam, P.; Adourian, A.; Böhm, M.; Van Veldhuisen, D.J.; Komajda, M.; et al. Galectin-3 predicts response to statin therapy in the controlled rosuvastatin multinational trial in heart failure (CORONA). Eur. Hear. J. 2012, 33, 2290–2296. [Google Scholar] [CrossRef]
- Sharim, J.; Daniels, L.B. Soluble ST2 and Soluble Markers of Fibrosis: Emerging Roles for Prognosis and Guiding Therapy. Curr. Cardiol. Rep. 2020, 22, 41. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Sasso, B.L.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef]
- Eliaz, I.; Raz, A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients 2019, 11, 2619. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The Impact of Galectin-3 Inhibition on Aldosterone-Induced Cardiac and Renal Injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef]
- Vergaro, G.; Prud’Homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.-L.; Cohen-Solal, A.; Delcayre, C. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension 2016, 67, 606–612. [Google Scholar] [CrossRef]
- Lax, A.; Sanchez-Mas, J.; Asensio-Lopez, M.C.; Palacio, M.J.F.-D.; Caballero, L.; Garrido, I.P.; Pastor-Pérez, F.J.; Januzzi, J.L.; Pascual-Figal, D.A. Mineralocorticoid Receptor Antagonists Modulate Galectin-3 and Interleukin-33/ST2 Signaling in Left Ventricular Systolic Dysfunction after Acute Myocardial Infarction. JACC Heart Fail. 2015, 3, 50–58. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
