Cystatin F is a protein inhibitor of cysteine cathepsins that is found intracellularly in the lysosomal/endosomal pathway as well as extracellularly. The extracellular cystatin F can be internalised into bystander cells and can affect cysteine cathepsin activity in recipient cells. In cytotoxic lymphocytes, extracellular cystatin F after internalisation decreases their cytotoxicity by affecting the activation of granzymes, effector molecules of the perforin/granzyme pathway.
- cystatin F
- cathepsins
- granzymes
- perforin
- TALL-104
- cytotoxic lymphocytes
1. Introduction
Cytotoxic T lymphocytes CD8+ (CTLs) and natural killer (NK) cells are crucial players in the immune response against virally infected cells and tumour cells [1][1]. The main target cell killing mechanism they use in this context is lytic granule exocytosis, which releases, most notably, perforin and granzymes. Granzymes are serine peptidases that trigger cell death upon entering the cell via the pore-forming protein perforin [1,2][1][2]. Both perforin and granzymes are synthesised as inactive precursors, which are proteolytically activated by cysteine cathepsins [3,4,5][3][4][5].
In cytotoxic lymphocytes, cathepsin C is the major progranzyme convertase, activating progranzymes by removing a dipeptide at their N-terminal end [4][4]. In the absence of cathepsin C, cathepsin H can act as an additional progranzyme convertase, at least for granzyme B [5][5]. Cysteine cathepsins also activate perforin precursors by cleaving their C-terminal ends [3,6][3][6], and cathepsin L has been suggested to play a major role in this process [6,7][6][7].
In immune cells, cystatin F, an endogenous protein inhibitor, was described to play an important role in regulating cysteine cathepsin activity [8][8]. Cystatin F belongs to type II cystatins; however, unlike other members that are mainly secreted, cystatin F is also localised intracellularly in lysosomal/endosomal compartments [9][9]. In addition, its inhibitory activity is tightly regulated as, after synthesis, it forms disulphide-linked dimers that do not inhibit cysteine cathepsins [10]. To be active inhibitors of cysteine cathepsins, cystatin F dimers must monomerise. This requires strong reducing conditions [10][10]; however, the process is highly facilitated by the truncation of the N-terminal end [11][11]. In addition, N-terminal truncation significantly changes the inhibitory profile of cystatin F. Whereas the full-length monomeric form does not inhibit cathepsin C, N-terminally truncated cystatin F is a potent inhibitor of cathepsin C with an equilibrium inhibitory constant in the nanomolar range [11][11]. Moreover, cystatin F can be secreted and internalised by bystander cells[12] [12] in which its intracellular localisation and uptake is driven by its glycosylation [12,13,14][12][13][14].
In cytotoxic cells, cystatin F is localised in cytotoxic granules in which it co-localises with its target cathepsins C, H, and L [11,15,16][11][15][16] indicating that cystatin F regulates their activities and thus the activation of the effector molecules of the perforin/granzyme pathway [8,17][8][17]. Indeed, it was demonstrated that extracellular cystatin F decreases the activities of granzymes A and B as well as the cytotoxicity of NK-92 and primary human NK cells [13][13]. In addition, both dimeric and monomeric cystatin F levels were increased in split anergic primary NK cells [15][15], a state characterised by decreased cytotoxicity and increased cytokine secretion [18,19][18][19]. Similarly, cystatin F levels were increased while the activities of cathepsins C, H, and L and granzyme B were decreased in the cytotoxic T CD8+ cell line (TALL-104) when a hyporesponsive anergic state was induced by ionomycin or transforming growth factor beta [16][16]. Likewise, the overexpression of cystatin F in mouse CTLs was shown to attenuate cathepsin C activity [11][11], and internalisation of cystatin F secreted from mouse CTLs into cystatin F-null mouse CTLs was also demonstrated [14][14]. However, whether extracellular cystatin F can directly affect cysteine cathepsin activity in recipient CTLs and decrease their cytotoxicity has not been demonstrated yet.
2. Effect of Internalised Cystatin F on Target Cathepsins in Cytotoxic T Lymphocytes
We provide evidence that extracellular cystatin F can be fully internalised into cytotoxic TALL-104 cells, and, as in NK cells, can decrease their cytotoxicity by inhibiting granzyme A and B activity (Figure 71). A similar result was obtained using primary human CTLs. Furthermore, we show that cystatin F secreted from target cells has a protective role against the cytotoxic action of TALL-104 cells.
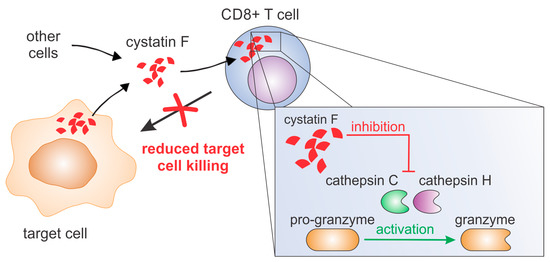
Figure 71. Cystatin F can be secreted from several cell types, such as monocytes, dendritic cells, mast cells or cancer cells. After internalisation into cytotoxic T cells it inhibits activities of cathepsins C and H and consequently activation of granzymes. This leads to decreased killing of target cells.
Extracellular cystatin F was demonstrated to be internalised by several cell types, including human HeLa and HEK-293 cell lines[13][14] [13,14] and murine fibroblast cell line L929 [14][14], murine bone marrow-derived macrophages [12], [12] bone marrow-derived dendritic cells from cystatin F knock-out mice [12][12], and CTLs and splenocytes from cystatin F knock-out mice [14][14]. All of these studies demonstrated uptake by cells that do not express endogenous cystatin F. In NK cells that express endogenous cystatin F, extracellular cystatin F uptake was demonstrated to potentiate the suppressive function of endogenous cystatin F regarding granzyme function and cell cytotoxicity [13][13]. As the internalised cystatin F in cystatin F-expressing cells must compete with endogenous intracellular cystatin F, the target peptidases might differ between internalised and endogenous cystatin F. However, here we demonstrate that the target of internalised extracellular cystatin F in TALL-104 cells is also cathepsin C, similar to endogenous cystatin F that also forms immune complexes with cathepsin C [11,16][11][16].
To confirm the effect of internalised cystatin F on target cathepsins, we analysed cathepsin C, H, and L activity in TALL-104 cells and found that they were significantly reduced. We also compared the effect between full-length and N-terminally truncated cystatin F on their activities. N-terminally truncated cystatin F is a monomeric form and can immediately interact with cathepsins after internalisation. Conversely, full-length cystatin F forms inactive dimers that must first be processed at the N-terminus and monomerise to become active inhibitors [10,11][10][11]. Both forms, full-length and N-terminally truncated, showed similar inhibitory activity, suggesting efficient activation of the full-length cystatin F upon internalisation (Figure S1). Besides direct binding and inhibition, cystatin F might decrease cathepsin C activity via inhibiting cathepsin L, which is involved in processing of pro-cathepsin C to its mature form. In HeLa cells that do not express endogenous cystatin F, transfection with full-length cystatin F resulted in lower cathepsin C processing. Nevertheless, evaluating the levels of precursor and mature forms of cathepsin C in TALL-104 cells after incubation with either full-length or N-terminally truncated cystatin F revealed no effect on cathepsin C processing. It is possible that in TALL-104 cells another peptidase, which is not inhibited by cystatin F, activates cathepsin C [20,23][20][23]. Another explanation for the observed difference in cathepsin C maturation between HeLa and TALL-104 cells is that HeLa cells were transfected with cystatin F while TALL-104 cells were pretreated with cystatin F; it is likely that internalised cystatin F is solely targeted to cellular compartments in which cathepsin C is already found in the activated form and could therefore directly inhibit cathepsin C and not affect its maturation. Furthermore, we tested the effect of cystatin F on perforin processing through inhibition of cathepsin L [7][7]. However, in TALL-104 cells, full-length or N-terminally truncated cystatin F did not affect perforin processing. Other peptidases probably compensate decreased cathepsin L activity, as substantial redundancy among peptidases regarding perforin activation has been demonstrated [6][6]. In contrast to perforin, full-length cystatin F caused a marked decrease in granzyme A and B activity in TALL-104 cells and primary human CTLs. Furthermore, the effect on granzyme A and B activity of N-terminally truncated cystatin F was significantly more pronounced than that of the full-length form in TALL-104 cells. This decrease in granzyme activity had functional consequences, i.e., decreased TALL-104 cell cytotoxicity against K-562 target cells and decreased cytotoxicity of SEA/SEB-activated primary human CTLs against SEA/SEB-pulsed Raji target cells. Treatment with both full-length and N-terminally truncated cystatin F forms led to a significant decrease in TALL-104 cell cytotoxicity. This is consistent with the results obtained for both NK-92 cell line and primary NK cells [13][13]. However, the difference in the effect on TALL-104 cell cytotoxicity of N-terminally truncated cystatin F compared to wild-type was significantly less pronounced compared to the difference observed in NK-92 cell line and primary NK cells [13][13]. A possible explanation is that TALL-104 cells might be more efficient in cystatin F activation due to, higher levels of cystatin F-activating peptidase.
Finally, we investigated whether cystatin F in target cells could confer protection against CTL killing. Indeed, cystatin F-pretreated target K-562 cells were killed less efficiently by TALL-104 cells. In the timeframe of the cytotoxicity assay, cystatin F-loaded target K-562 cells released a large portion of internalised cystatin F. Therefore, we propose that target cells may protect themselves from CTL killing by secreting cystatin F, which is subsequently internalised into cytotoxic cells, in which it inhibits the activation of granzymes that are crucial for efficient target cell killing. A protective role of cystatin F in target cells might be especially important in tumour microenvironments in which cystatin F could be exploited by tumour cells to evade the anti-tumour immune response. Indeed, up-regulated cystatin F expression in tumour tissue was demonstrated in patients with colorectal cancer, and was even correlated with liver metastasis and worse prognoses [24]. A similar molecular mechanism was demonstrated for serpinB9, an inhibitor that can directly inhibit granzyme B; tumour cells were shown to protect themselves from cytotoxic lymphocytes by up-regulating serpinB9 [25][25]. Tumour cells could also internalise cystatin F present in tumour microenvironments into which it could be secreted by several other cell types, especially immune cells such as dendritic cells, monocytes or even cytotoxic lymphocytes [13][13]. To conclude, our results suggest that cystatin F is an important molecular mediator in tumour microenvironments and is responsible for shaping the anti-tumour immune response and function of cytotoxic lymphocytes.
Reference (we'll rearrange the references after you submitted it)
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747.
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5047–5056.
- Uellner, R.; Zvelebil, M.J.; Hopkins, J.; Jones, J.; MacDougall, L.K.; Morgan, B.P.; Podack, E.; Waterfield, M.D.; Griffiths, G.M. Perforin is activated by a proteolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 1997, 16, 7287–7296.
- Sutton, V.R.; Waterhouse, N.J.; Browne, K.A.; Sedelies, K.; Ciccone, A.; Anthony, D.; Koskinen, A.; Mullbacher, A.; Trapani, J.A. Residual active granzyme B in cathepsin C-null lymphocytes is sufficient for perforin-dependent target cell apoptosis. J. Cell Biol. 2007, 176, 425–433.
- D’Angelo, M.E.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.A.; Sutton, V.R. Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519.
- House, I.G.; House, C.M.; Brennan, A.J.; Gilan, O.; Dawson, M.A.; Whisstock, J.C.; Law, R.H.; Trapani, J.A.; Voskoboinik, I. Regulation of perforin activation and pre-synaptic toxicity through C-terminal glycosylation. EMBO Rep. 2017, 18, 1775–1785.
- Konjar, S.; Sutton, V.R.; Hoves, S.; Repnik, U.; Yagita, H.; Reinheckel, T.; Peters, C.; Turk, V.; Turk, B.; Trapani, J.A.; et al. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology 2010, 131, 257–267.
- Kos, J.; Nanut, M.P.; Prunk, M.; Sabotič, J.; Dautović, E.; Jewett, A. Cystatin F as a regulator of immune cell cytotoxicity. Cancer Immunol. Immunother. CII 2018, 67, 1931–1938.
- Nathanson, C.-M.; Wassélius, J.; Wallin, H.; Abrahamson, M. Regulated expression and intracellular localization of cystatin F in human U937 cells. Eur. J. Biochem. 2002, 269, 5502–5511.
- Langerholc, T.; Zavasnik-Bergant, V.; Turk, B.; Turk, V.; Abrahamson, M.; Kos, J. Inhibitory properties of cystatin F and its localization in U937 promonocyte cells. FEBS J. 2005, 272, 1535–1545.
- Hamilton, G.; Colbert, J.D.; Schuettelkopf, A.W.; Watts, C. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J. 2008, 27, 499–508.
- Colbert, J.D.; Matthews, S.P.; Kos, J.; Watts, C. Internalization of exogenous cystatin F supresses cysteine proteases and induces the accumulation of single-chain cathepsin L by multiple mechanisms. J. Biol. Chem. 2011, 286, 42082–42090.
- Perišić Nanut, M.; Sabotič, J.; Švajger, U.; Jewett, A.; Kos, J. Cystatin F affects natural killer cell cytotoxicity. Front. Immunol. 2017, 8, 1459.
- Colbert, J.D.; Plechanovová, A.; Watts, C. Glycosylation directs targeting and activation of cystatin F from intracellular and extracellular sources. Traffic 2009, 10, 425–437.
- Magister, Š.; Tseng, H.-C.; Bui, V.T.; Kos, J.; Jewett, A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget 2015, 6, 22310–22327.
- Prunk, M.; Perišić Nanut, M.; Sabotič, J.; Švajger, U.; Kos, J. Increased cystatin F levels correlate with decreased cytotoxicity of cytotoxic T cells. Radiol. Oncol. 2019, 53, 57–68.
- Prunk, M.; Nanut, M.P.; Sabotic, J.; Kos, J. Cystatins, cysteine peptidase inhibitors, as regulators of immune cell cytotoxicity. Period. Biol. 2016, 118, 353–362.
- Kaur, K.; Nanut, M.P.; Ko, M.-W.; Safaie, T.; Kos, J.; Jewett, A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: Strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr. Opin. Immunol. 2018, 51, 170–180.
- Jewett, A.; Kos, J.; Fong, Y.; Ko, M.-W.; Safaei, T.; Perišić Nanut, M.; Kaur, K. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin. Cancer Biol. 2018, 53, 178–188.
- Dahl, S.W.; Halkier, T.; Lauritzen, C.; Dolenc, I.; Pedersen, J.; Turk, V.; Turk, B. Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 2001, 40, 1671–1678.
- Prunk, M.; Perišić Nanut, M.; Kos, J. Incubation of TALL-104 cells with cystatin F for 4 days. (unpublished data).
- Perišić Nanut, M.; Sabotič, J.; Jewett, A.; Kos, J. Cysteine cathepsins as regulators of the cytotoxicity of NK and T cells. Front. Immunol. 2014, 5, 616.
- Mallen-St. Clair, J.; Shi, G.-P.; Sutherland, R.E.; Chapman, H.A.; Caughey, G.H.; Wolters, P.J. Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol. Chem. 2006, 387, 1143–1146.
- Utsunomiya, T.; Hara, Y.; Kataoka, A.; Morita, M.; Arakawa, H.; Mori, M.; Nishimura, S. Cystatin-like metastasis-associated protein mRNA expression in human colorectal cancer is associated with both liver metastasis and patient survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2591–2594.
- Chen, J.; Cao, Y.; Markelc, B.; Kaeppler, J.; Vermeer, J.A.; Muschel, R.J. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Investig. 2019, 129, 4224–4238.
References
- Trapani, J.A.; Smyth, M.J. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2002, 2, 735–747.
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5047–5056.
- Uellner, R.; Zvelebil, M.J.; Hopkins, J.; Jones, J.; MacDougall, L.K.; Morgan, B.P.; Podack, E.; Waterfield, M.D.; Griffiths, G.M. Perforin is activated by a proteolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 1997, 16, 7287–7296.
- Sutton, V.R.; Waterhouse, N.J.; Browne, K.A.; Sedelies, K.; Ciccone, A.; Anthony, D.; Koskinen, A.; Mullbacher, A.; Trapani, J.A. Residual active granzyme B in cathepsin C-null lymphocytes is sufficient for perforin-dependent target cell apoptosis. J. Cell Biol. 2007, 176, 425–433.
- D’Angelo, M.E.; Bird, P.I.; Peters, C.; Reinheckel, T.; Trapani, J.A.; Sutton, V.R. Cathepsin H is an additional convertase of pro-granzyme B. J. Biol. Chem. 2010, 285, 20514–20519.
- House, I.G.; House, C.M.; Brennan, A.J.; Gilan, O.; Dawson, M.A.; Whisstock, J.C.; Law, R.H.; Trapani, J.A.; Voskoboinik, I. Regulation of perforin activation and pre-synaptic toxicity through C-terminal glycosylation. EMBO Rep. 2017, 18, 1775–1785.
- Konjar, S.; Sutton, V.R.; Hoves, S.; Repnik, U.; Yagita, H.; Reinheckel, T.; Peters, C.; Turk, V.; Turk, B.; Trapani, J.A.; et al. Human and mouse perforin are processed in part through cleavage by the lysosomal cysteine proteinase cathepsin L. Immunology 2010, 131, 257–267.
- Kos, J.; Nanut, M.P.; Prunk, M.; Sabotič, J.; Dautović, E.; Jewett, A. Cystatin F as a regulator of immune cell cytotoxicity. Cancer Immunol. Immunother. CII 2018, 67, 1931–1938.
- Nathanson, C.-M.; Wassélius, J.; Wallin, H.; Abrahamson, M. Regulated expression and intracellular localization of cystatin F in human U937 cells. Eur. J. Biochem. 2002, 269, 5502–5511.
- Langerholc, T.; Zavasnik-Bergant, V.; Turk, B.; Turk, V.; Abrahamson, M.; Kos, J. Inhibitory properties of cystatin F and its localization in U937 promonocyte cells. FEBS J. 2005, 272, 1535–1545.
- Hamilton, G.; Colbert, J.D.; Schuettelkopf, A.W.; Watts, C. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J. 2008, 27, 499–508.
- Colbert, J.D.; Matthews, S.P.; Kos, J.; Watts, C. Internalization of exogenous cystatin F supresses cysteine proteases and induces the accumulation of single-chain cathepsin L by multiple mechanisms. J. Biol. Chem. 2011, 286, 42082–42090.
- Perišić Nanut, M.; Sabotič, J.; Švajger, U.; Jewett, A.; Kos, J. Cystatin F affects natural killer cell cytotoxicity. Front. Immunol. 2017, 8, 1459.
- Colbert, J.D.; Plechanovová, A.; Watts, C. Glycosylation directs targeting and activation of cystatin F from intracellular and extracellular sources. Traffic 2009, 10, 425–437.
- Magister, Š.; Tseng, H.-C.; Bui, V.T.; Kos, J.; Jewett, A. Regulation of split anergy in natural killer cells by inhibition of cathepsins C and H and cystatin F. Oncotarget 2015, 6, 22310–22327.
- Prunk, M.; Perišić Nanut, M.; Sabotič, J.; Švajger, U.; Kos, J. Increased cystatin F levels correlate with decreased cytotoxicity of cytotoxic T cells. Radiol. Oncol. 2019, 53, 57–68.
- Prunk, M.; Nanut, M.P.; Sabotic, J.; Kos, J. Cystatins, cysteine peptidase inhibitors, as regulators of immune cell cytotoxicity. Period. Biol. 2016, 118, 353–362.
- Kaur, K.; Nanut, M.P.; Ko, M.-W.; Safaie, T.; Kos, J.; Jewett, A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: Strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr. Opin. Immunol. 2018, 51, 170–180.
- Jewett, A.; Kos, J.; Fong, Y.; Ko, M.-W.; Safaei, T.; Perišić Nanut, M.; Kaur, K. NK cells shape pancreatic and oral tumor microenvironments; role in inhibition of tumor growth and metastasis. Semin. Cancer Biol. 2018, 53, 178–188.
- Dahl, S.W.; Halkier, T.; Lauritzen, C.; Dolenc, I.; Pedersen, J.; Turk, V.; Turk, B. Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 2001, 40, 1671–1678.
- Prunk, M.; Perišić Nanut, M.; Kos, J. Incubation of TALL-104 cells with cystatin F for 4 days. (unpublished data).
- Perišić Nanut, M.; Sabotič, J.; Jewett, A.; Kos, J. Cysteine cathepsins as regulators of the cytotoxicity of NK and T cells. Front. Immunol. 2014, 5, 616.
- Mallen-St. Clair, J.; Shi, G.-P.; Sutherland, R.E.; Chapman, H.A.; Caughey, G.H.; Wolters, P.J. Cathepsins L and S are not required for activation of dipeptidyl peptidase I (cathepsin C) in mice. Biol. Chem. 2006, 387, 1143–1146.
- Utsunomiya, T.; Hara, Y.; Kataoka, A.; Morita, M.; Arakawa, H.; Mori, M.; Nishimura, S. Cystatin-like metastasis-associated protein mRNA expression in human colorectal cancer is associated with both liver metastasis and patient survival. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2591–2594.
- Chen, J.; Cao, Y.; Markelc, B.; Kaeppler, J.; Vermeer, J.A.; Muschel, R.J. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Investig. 2019, 129, 4224–4238.
