This entry is dealing with fluorescein and its derivatives and focuses on their uses as pH-sensor in a biological context. It gathers chemical properties of the fluorescein family of compounds combined to biological issues.
- fluorescein
- derivatives
- ph
- sensor
- properties
1. Introduction
In chemistry, potential of hydrogen, or pH, is a data describing the acidity or the basicity of a medium [1]. Logarithmically obtained from H+ ion concentration, pH is one of the main physical characteristics used to describe an aqueous solution. Since the development of pH-meters, its measurement became inevitable across scientific fields, such as drinking water [2], industrial waste [3][4][3,4], global health [5], and agronomy [6]. As acidic or basic compounds are continuously released as outputs of cellular life, pH monitoring offered a unique opportunity to easily acquired data from biologic systems [7]. The relevance of pH in biological systems can be observed at different scales: the pH of biological fluids is well described and its regulation essential to the proper function of organs, since abnormal values are both the sign and cause of disease developments. pH regulation within biological systems relies on a sensitive equilibrium, called pH homeostasis. At the organism level, pH regulation is performed by the lungs through the elimination of CO2 and the kidney through the filtration of the HCO3− ion. In cells, where organic acids, such as lactic, pyruvic, or beta-hydroxybutyrate acids are produced along metabolic pathways, some membrane proteins compensate the decrease of pH by transporting protons outside the cytosol [8]. Thus, cellular pH monitoring leads to the understanding of key milestones within cells, such as proliferation [9], ion transport [10], or carcinogenesis [11]. For example, unchecked biological processes occurring in malignant cells release a consequent amount of acid derivatives, leading to a pH decrease in tumoral tissues [12]. In microbiology, proliferations of aerobic bacteria also lead to a massive production of acidic metabolites, which quickly induce pH variations in medium [13]. Whereas global pH in cytosol has to be regulated, each organelle is fully effective in specific ionic environments, which are often correlated to local functions [14]. For example, the average pH in lysosomes, Golgi network, and mitochondria are 4.7, 6.7, and 8 respectively. Thus, pH monitoring of each organelle has helped to determine their function. At an atomic scale, all metabolism pathways are directly correlated to pH since enzymatic activities and kinetics depend on ionic environments. Another common example is the classification of essential amino acids, according their acidic or basic trends. Thus, pH is an important physicochemical datum, which can attest of biological activities at different scales. In order to monitor these fluctuations without any physical contact, fluorescent pH-sensitive probes were developed during the last century. Under specific light, these molecular probes re-emit photons at another wavelength for which related intensity depends on the surrounding pH [7]. Since optical devices are continuously becoming more precise, especially with the broad dissemination of optic fibers, pH-sensitive molecular probes are recurrent across biological studies.
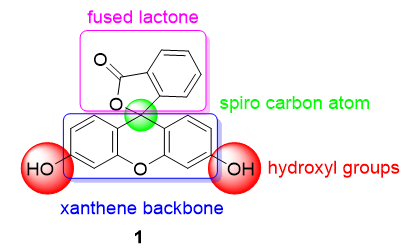
One of the most used pH probes is fluorescein 1; its chemical structure is composed of a tricyclic xanthene flanked by two hydroxyl groups and a bicyclic fused lactone fragment linked by a spiro carbon atom (Figure 1).
Figure 1. Chemical structure of fluorescein 1.
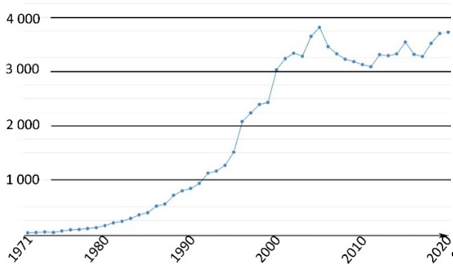
This family of molecules was discovered in 1871 by Adolf von Baeyer, and has become one of most ubiquitous probes in biological studies, because of its intense fluorescence, reversible pH sensitivity, chemical stability, and lack of cytotoxicity at working concentrations. For years, fluorescein 1 has been used as a starting material to create novel fluorescent probes revealing specific biological activities such as enzymatic cleavage [15]. Fluorescein 1 is still the focus of interest from the scientific community for its intense fluorescence and its sensitivity to pH variations around neutral domain [16]. As most biological systems are fully effective at physiological conditions (pH~7.3), fluorescein 1 became a benchmark for monitoring pH fluctuations in cell cultures. Indeed, every year, a substantial amount of new articles describes biological studies using fluorescein 1 as pH-sensitive probe (Figure 2) [17]. In this review, we propose an overview of fluorescein 1 and its main derivatives complemented by a survey of recent studies using fluorescein 1 as pH sensors for biological applications.
Figure 2. Number of articles with keywords “fluorescein, pH, probe, biology” during last fifty years [17].
2. Fluorescein and Derivatives as Notorious pH Sensors
2.1. Fluorescein: Synthesis and Properties
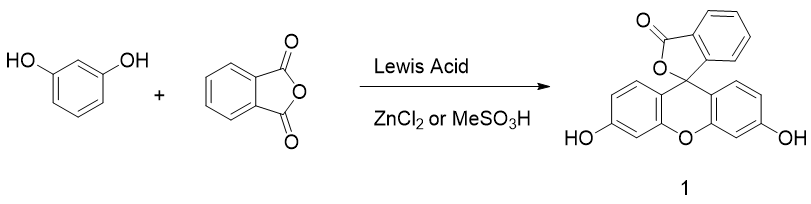
Originally obtained by condensing phtalic anhydride with phenol in acidic conditions by Von Baeyer, this preparation of fluorescein 1 is nowadays based on Friedel–Crafts reactions (Figure 3). Thus, the fluorophore can be easily obtained by mixing phtalic anhydride, resorcinol and ZnCl2 or methanesulfonic acid [18][19][18,19]. Large scale manufactured, fluorescein 1 has been included in the World Health Organization (WHO)’s model list of essential medicines [20] for its biological applications, but it is also used in other fields, such as petrochemistry for leak detection and cosmetic formulations.
Figure 3. General synthetic pathway to fluorescein.
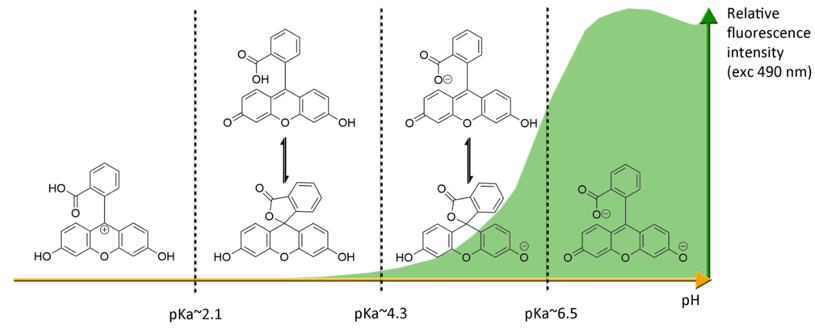
Under basic conditions (pH > 8), fluorescein 1 absorbs blue light with a maxima absorption peak around 490 nm, and emits a green light around 515 nm [21]. Due to phenolic fragments and equilibrium between a carboxylic function and a lactone, ionic charge and chemical structure of fluorescein evolve depending on the surrounding pH, leading to fluctuations of photophysical properties (Figure 4) [22]. Whereas the fluorescence quantum yield under an excitation at 490 nm is very high under basic conditions (ΦF: 0.95 in NaOH 0.1 M), an acidification of the solution progressively leads to the fluorescence extinction [23]. This fluctuation is due to the transition of the di-anionic form of fluorescein into an anionic equilibrium, which has a lower absorbance associated to a blue-shifting [23].
Figure 4. Ionic forms of fluorescein 1 according the pH domains and their relative fluorescence intensities. At neutral pH and under excitation at 490 nm, the most fluorescent di-anionic form of fluorescein takes prominence over other forms. Below pH = pKa~6.4, mono-anionic fluorescein displays a blue-shifted absorption followed by drastic decrease of fluorescence. At even lower pH, neutral and further cationic forms of fluorescein becomes non-fluorescent under irradiation at 490 nm.
The most problematic drawback of fluorescein is its photobleaching when exposed to light [24]. Indeed, the fluorescent emission from this probe progressively decreases under intense light irradiations, which is an issue since pH monitoring is based on this intensity, and repeated measurements cause the signal to fade. Because of this photobleaching, all fluorescein derivatives must be stored in the dark, and experiments using this probe have to occur quickly. Spectral bands of fluorescein can also induce issues with some optical systems. Depending on the purpose of the application, its relatively broad fluorescent emission can be considered as an advantage (for example, in case of the Förster resonance energy transfer (FRET) system), but also as a drawback for multi fluorescent probing experiences. Fluorescein presents also a weak Stokes shift, the interval between highest absorption and emission wavelength, which is a potential issue for devices using a single optical path, such as entry-level plate readers. Like most intense fluorescent probes, self-quenching can occur in case of aggregation (ACQ) or high degrees of surface substitution [25]. Hopefully, due to low the concentration used in biological systems, this drawback has little relevance.
2.2. Most Used Fluorescein Derivatives and Their Properties
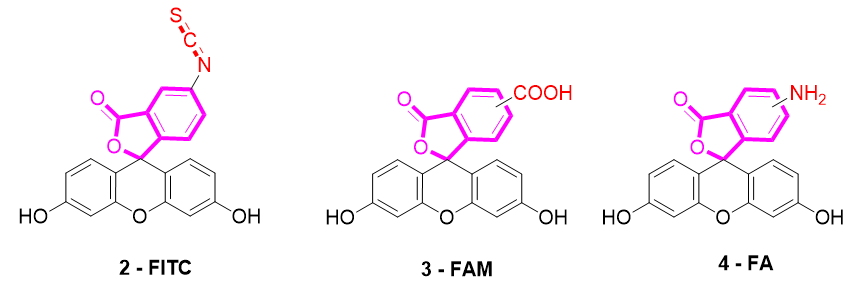
Several fluorescein derivatives have been developed in order to fit with the downstream applications. The best-known derivatives are based on the modification of benzo-fused lactone moiety in order to add reactive chemical functions such as isothiocyanate, carboxylic acid, or amine. The outcoming probes are named fluorescein isothiocyanate (FITC) (Figure 5-2), carboxyfluorescein (5(6)-FAM or CF) (Figure 5-3), and fluoresceinamine (FA) (Figure 5-4), respectively.
Figure 5. Most used reactive derivatives of fluorescein.
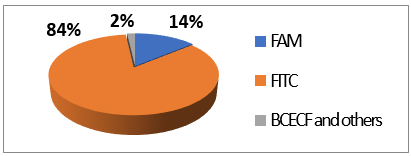
FITC 2 is one of most used derivatives due to its properties and good reactivity for conjugation. In comparison to fluorescein, FITC 2 presents a slight decrease of fluorescence quantum yield (ΦF (excitation: 500 nm): 0.75 in 10 mM Phosphate-Buffered Saline) [26]. However, in comparison with other fluorophores, FITC 2 still has an intense fluorescence and can be used as a pH-sensitive probe around the neutral domain. FITC 2 can be easily associated to any kind of chemical structures bearing an amine fragment, such as fluorophores [27], targeting agents [28], proteins [29], polymers [30], or nanoparticles [31]. Resulting bioconjugation linkage is based on a thiourea function for which stability depends on physiochemical surroundings due to its strong H-bond interactions [32][33][34][32–34]. Using such derivatives offers a unique opportunity to create specific pH sensors, and particularly, for biological systems where all proteins are bearing a terminal amine. Even if FITC 2 is still the most prolific derivative in literature (Figure 6), new fluorescein reactive derivatives are still described with optimized properties [35].
Figure 6.
Average repartition of used fluorescein derivatives in literature the last decade [17].
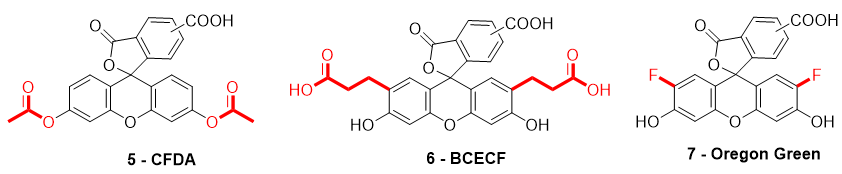
When fluorescein was used for intracellular pH (pHin) monitoring, a high leakage rate appeared from the cells, which made accurate pH determination difficult. Fluorescein 1 was then replaced by carboxyfluorescein 3 (FAM or CF) as the main pHin probe, since its main advantage was its low leaking rate through cell membranes. Indeed, due to its additional carboxylic acid function, the supplementary anionic charge significantly reduces solubility of FAM 3 in lipid structures composing cell membranes [36] Moreover, FAM 3 has similar photophysical properties to FITC 2 [26]. Thus, FAM 3 has become one of the most used probes for characterization of tumoral tissue [37]. FAM 3 can also be used for preparation of bioconjugates using carbodiimide/NHS activation. In this case, resulting amide function is stable due to its omnipresence in biological systems. Diacetate derivatives of fluorescein and FAM 3, respectively named fluorescein di-acetate (FDA) and carboxyfluorescein di-acetate (CFDA) (Figure 7-5), were also been used across several studies [38][39][38,39]. Di-ester analogs present a quenching of the fluorescence, but, under esterase intracellular activities, emissions are restored [40]. Thus, these derivatives are used to estimate intracellular enzymatic activities [41]. Moreover, in comparison of FAM 3, which is used for its weak membrane permeability, diacetate derivatives are cell permeable probes. Thus, CFDA 5 is one of best pHin sensors because FAM 3 is directly released under intracellular enzymatic activities and is not able to leak outside cells.
Figure 7. Different fluorescein derivatives used as pH-sensitive probes.
While FITC 2 and FAM 3 are the most prominent fluorescein derivatives in the literature, other probes were developed for specific and optimized applications at physiological pH. Introduced by Roger Tsien in 1982, 2′,7′-bis-(2-carboxyethyl)-carboxyfluorescein (BCECF) (Figure 7-6) was developed in order to fit more precisely with pH variation around neutral domain [42]. Indeed, due to its pKa near 7, weak acidification of medium directly induces a significant reduction of fluorescence intensity. Moreover, the absorption spectrum of BCECF 6 possesses an isosbestic point, where its absorbance is pH-independent. This property allows the establishment of ratiometric analyses for which a ratio of fluorescence is calculated from excitations at two different wavelengths (generally at maxima and isosbestic point). These protocols greatly overcome common hurdles that are dye loading, leakage, optical imprecisions, or photobleaching [43].
In order to fit with acidic organelles, present in yeast [44] for example, fluorinated derivatives of fluorescein, such as Oregon Green 7, have been prepared for acidic pH monitoring (Figure 7-7) [45]. Introduction of electron-withdrawing atoms within the xanthene structure leads to a decrease of pKa around 4.7. Then, pH-sensitivity of such compounds is suitable with cellular components operating at pH around 5.
References
- Jensen, W.B. The Symbol for pH. Chem. Educ. 2004, 81, 21, doi:10.1021/ed081p21.
- Dietrich, A.M.; Burlingame, G.A. Critical Review and Rethinking of USEPA Secondary Standards for Maintaining Organoleptic Quality of Drinking Water. Sci. Technol. 2015, 49, 708–720, doi:10.1021/es504403t.
- Al-Hameedi, A.T.T.; Alkinani, H.H.; Albazzaz, H.W.; Dunn-Norman, S.; Alkhamis, M.M. Insights into the applications of waste materials in the oil and gas industry: state of the art review, availability, cost analysis, and classification. Pet. Explor. Prod. Technol. 2020, 10, 2137–2151, doi:10.1007/s13202-020-00865-w.
- Mohammad, N.; Alam, M.Z.; Kabbashi, N.A.; Ahsan, A. Effective composting of oil palm industrial waste by filamentous fungi: A review. Conserv. Recycl. 2012, 58, 69–78, doi:10.1016/j.resconrec.2011.10.009.
- Zappulla, D. The CO2 hypothesis—The stress of global warming on human health: pH homeostasis, the linkage between breathing and feeding via CO2 In Air Pollution: Sources, Prevention and Health Effects; Book from Nova Science Publishers (USA). 2013; pp. 293–313.
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417, doi:10.1007/s11104-012-1429-7.
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Rev. 2010, 110, 2709–2728, doi:10.1021/cr900249z.
- Aoi, W.; Marunaka, Y. Importance of pH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. BioMed Res. Int. 2014, 2014, 1–8, doi:10.1155/2014/598986.
- Tamagaki, T.; Sawada, S.; Imamura, H.; Tada, Y.; Yamasaki, S.; Toratani, A.; Sato, T.; Komatsu, S.; Akamatsu, N.; Yamagami, M.; et al. Effects of high-density lipoproteins on intracellular pH and proliferation of human vascular endothelial cells. Atherosclerosis 1996, 123, 73–82, doi:10.1016/0021-9150(95)05774-9.
- Di Sario, A.; Bendia, E.; Omenetti, A.; De Minicis, S.; Marzioni, M.; Kleemann, H.W.; Candelaresi, C.; Saccomanno, S.; Alpini, G.; Benedetti, A. Selective inhibition of ion transport mechanisms regulating intracellular pH reduces proliferation and induces apoptosis in cholangiocarcinoma cells. Liver Dis. 2007, 39, 60–69, doi:10.1016/j.dld.2006.07.013.
- Bohloli, M.; Atashi, A.; Soleimani, M.; Kaviani, S.; Anbarlou, A. Investigating Effects of Acidic pH on Proliferation, Invasion and Drug-Induced Apoptosis in Lymphoblastic Leukemia. Cancer Microenviron 2016, 9, 119–126, doi:10.1007/s12307-016-0187-0.
- Stubbs, M.; McSheehy, P.M.J.; Griffiths, J.R. Causes and consequences of acidic pH in tumors: A magnetic resonance study. Enzym. Regul. 1999, 39, 13–30, doi:10.1016/S0065-2571(98)00018-1.
- Vereecken, K.M.; Van Impe, J.F. Analysis and practical implementation of a model for combined growth and metabolite production of lactic acid bacteria. J. Food Microbiol. 2002, 73, 239–250, doi:10.1016/S0168-1605(01)00641-9.
- Theillet, F.-X.; Binolfi, A.; Frembgen-Kesner, T.; Hingorani, K.; Sarkar, M.; Kyne, C.; Li, C.; Crowley, P.B.; Gierasch, L.; Pielak, G.J.; et al. Physicochemical Properties of Cells and Their Effects on Intrinsically Disordered Proteins (IDPs). Rev. 2014, 114, 6661–6714, doi:10.1021/cr400695p.
- Gaspar, M.L.; Cabello, M.N.; Pollero, R.; Aon, M.A. Fluorescein Diacetate Hydrolysis as a Measure of Fungal Biomass in Soil. Microbiol. 2001, 42, 339–344, doi:10.1007/s002840010226.
- Sjöback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Acta. A. Mol. Biomol. Spectrosc. 1995, 51, L7–L21, doi:10.1016/0584-8539(95)01421-P.
- Available online: www.dimensions.ai (accessed on 10 October 2020).
- Brandt, R.; Keston, A.S. Synthesis of diacetyldichlorofluorescin: A stable reagent for fluorometric analysis. Biochem. 1965, 11, 6–9, doi:10.1016/0003-2697(65)90035-7.
- Ueno, Y.; Jiao, G.-S.; Burgess, K. Preparation of 5- and 6-Carboxyfluorescein. Synthesis 2004, 2004, 2591–2593, doi:10.1055/s-2004-829194.
- WHO Model Lists of Essential Medicines. Available online: http://www.who.int/medicines/publications/essentialmedicines/en/ (accessed on 22 October 2020).
- Sun, W.-C.; Gee, K.R.; Klaubert, D.H.; Haugland, R.P. Synthesis of Fluorinated Fluoresceins. Org. Chem. 1997, 62, 6469–6475, doi:10.1021/jo9706178.
- Klonis, N.; Sawyer, W.H. Spectral properties of the prototropic forms of fluorescein in aqueous solution. Fluoresc. 1996, 6, 147–157, doi:10.1007/BF00732054.
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. Lumin. 1975, 10, 381–390, doi:10.1016/0022-2313(75)90003-4.
- Song, L.; Hennink, E.J.; Young, I.T.; Tanke, H.J. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. J. 1995, 68, 2588–2600, doi:10.1016/S0006-3495(95)80442-X.
- Feng, S.; Gong, S.; Feng, G. Aggregation-induced emission and solid fluorescence of fluorescein derivatives. Commun. 2020, 56, 2511–2513, doi:10.1039/C9CC09784H.
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. Fluoresc. 2014, 24, 819–826, doi:10.1007/s10895-014-1356-5.
- Zhang, Q.; Zhou, M. A profluorescent ratiometric probe for intracellular pH imaging. Talanta 2015, 131, 666–671, doi:10.1016/j.talanta.2014.08.039.
- Li, Y.; Fu, Y.; Zhang, H.; Song, J.; Yang, S. FITC-Labeled Alendronate as an In Vivo Bone pH Sensor. BioMed Res. Int. 2020, 2020, 1–9, doi:10.1155/2020/4012194.
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin, V. Immunol. Methods 1995, 184, 39–51, doi:10.1016/0022-1759(95)00072-I.
- Majoros, I.J.; Myc, A.; Thomas, T.; Mehta, C.B.; Baker, J.R. PAMAM Dendrimer-Based Multifunctional Conjugate for Cancer Therapy: Synthesis, Characterization, and Functionality. Biomacromolecules 2006, 7, 572–579, doi:10.1021/bm0506142.
- Lu, C.-W.; Hung, Y.; Hsiao, J.-K.; Yao, M.; Chung, T.-H.; Lin, Y.-S.; Wu, S.-H.; Hsu, S.-C.; Liu, H.-M.; Mou, C.-Y.; et al. Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling. Nano Lett. 2007, 7, 149–154, doi:10.1021/nl0624263.
- Shaw, W.H.R.; Walker, D.G. The Decomposition of Thiourea in Water Solutions. Am. Chem. Soc. 1956, 78, 5769–5772, doi:10.1021/ja01603a014.
- Sahu, S.; Rani Sahoo, P.; Patel, S.; Mishra, B.K. Oxidation of thiourea and substituted thioureas: A review. Sulfur Chem. 2011, 32, 171–197, doi:10.1080/17415993.2010.550294.
- Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Urea vs. thiourea in anion recognition. Biomol. Chem. 2005, 3, 1495–1500, doi:10.1039/B500123D.
- Petri, L.; Szijj, P.A.; Kelemen, Á.; Imre, T.; Gömöry, Á.; Lee, M.T.W.; Hegedűs, K.; Ábrányi-Balogh, P.; Chudasama, V.; Keserű, G.M. Cysteine specific bioconjugation with benzyl isothiocyanates. RSC Adv. 2020, 10, 14928–14936, doi:10.1039/D0RA02934C.
- Grimes, P.A.; Stone, R.A.; Laties, A.M.; Li, W. Carboxyfluorescein. A probe of the blood-ocular barriers with lower membrane permeability than fluorescein. Ophthalmol. Chic. Ill. 1960 1982, 100, 635–639, doi:10.1001/archopht.1982.01030030637022.
- Mordon, S.; Maunoury, V.; Devoisselle, J.M.; Abbas, Y.; Coustaud, D. Characterization of tumorous and normal tissue using a pH-sensitive fluorescence indicator (5,6-carboxyfluorescein) in vivo. Photochem. Photobiol. B 1992, 13, 307–314, doi:10.1016/1011-1344(92)85070-B.
- Parish, C.R.; Glidden, M.H.; Quah, B.J.C.; Warren, H.S. Use of the Intracellular Fluorescent Dye CFSE to Monitor Lymphocyte Migration and Proliferation. Protoc. Immunol. 2009, 84, 4.9.1–4.9.13, doi:10.1002/0471142735.im0409s84.
- Zhang, F.; Zhang, P.; Wang, E.; Su, F.; Qi, Y.; Bu, X.; Wang, Y. Fluorescein Diacetate Uptake to Determine the Viability of Human Fetal Cerebral Cortical Cells. Histochem. 1995, 70, 185–187, doi:10.3109/10520299509107310.
- Hoefel, D.; Grooby, W.L.; Monis, P.T.; Andrews, S.; Saint, C.P. A comparative study of carboxyfluorescein diacetate and carboxyfluorescein diacetate succinimidyl ester as indicators of bacterial activity. Microbiol. Methods 2003, 52, 379–388, doi:10.1016/S0167-7012(02)00207-5.
- Xiao, X.; Han, Z.; Chen, Y.; Liang, X.; Li, H.; Qian, Y. Optimization of FDA–PI method using flow cytometry to measure metabolic activity of the cyanobacteria, Microcystis aeruginosa. Chem. Earth Parts ABC 2011, 36, 424–429, doi:10.1016/j.pce.2010.03.028.
- Boens, N.; Qin, W.; Basarić, N.; Orte, A.; Talavera, E.M.; Alvarez-Pez, J.M. Photophysics of the Fluorescent pH Indicator BCECF. Phys. Chem. A 2006, 110, 9334–9343, doi:10.1021/jp0615712.
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Chem. Rev. 2019, 383, 82–103, doi:10.1016/j.ccr.2019.01.004.
- Wada, Y.; Anraku, Y. Chemiosmotic coupling of ion transport in the yeast vacuole: Its role in acidification inside organelles. Bioenerg. Biomembr. 1994, 26, 631–637, doi:10.1007/BF00831538.
- Delmotte, C.; Delmas, A. Synthesis and fluorescence properties of oregon green 514 labeled peptides. Med. Chem. Lett. 1999, 9, 2989–2994, doi:10.1016/S0960-894X(99)00512-0.