The term “osteoimmunology” was first used in 2000 by Choi et al. to define a new paradigm describing the crosstalk between the immune system and osteoclastogenesis [1]. The multiplicity of osteoimmunological disorders is due to the variety of stimuli responsible for the immune system activation.
- osteoimmunological disorders
- osteoimmunology
- osteoclasts
- osteoblasts
- bone diseases
- breast cancer
- osteoporosis
- rheumatoid arthritis
- osteoarthritis
- periodontitis
1. Introduction
The term “osteoimmunology” was first used in 2000 by Choi et al. to define a new paradigm describing the crosstalk between the immune system and osteoclastogenesis [1][1]. The multiplicity of osteoimmunological disorders is due to the variety of stimuli responsible for the immune system activation. In fact, adaptative and innate immunity could be induced by several pathological (bacteria, tissue injury) or physiological (tissue remodeling) processes. Despite this variety of stimuli, the associated response remains similar between diseases: a crosstalk between myeloid lineage, mesenchymal stem cells and the inflammatory microenvironment, which results mainly in both excessive bone and cartilage resorption, and chronic inflammation, but also in bone formation in some cases (Figure 1)[1][2][3][4][5][6].
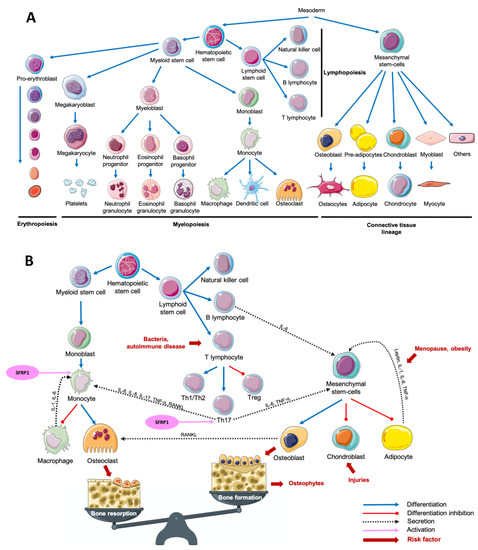
Figure 1. Main cell lineages (A) and their crosstalk in an osteoimmunological context (B). Abbreviations: IL = interleukin, RANKL = Receptor Activator of Nuclear factor Kappa-B Ligand, SFRP1 = Secreted Frizzled-Related Protein 1, Th = T helper, TNF-α = Tumor Necrosis Factor-alpha, Treg = T regulator.
2. Osteoporosis (OP)
Osteoporosis (OP) is a metabolic disease characterized by a loss of bone mass and an excessive fragility of bones due to an imbalance between bone resorption and bone formation. This multifactorial disease is notably due to an increased secretion of pro-inflammatory cytokines and adipokines, inducing an excessive osteoclastogenesis. Estrogen deprivation after menopause is an important OP risk factor. Estradiol serum level is inversely proportional to the risk of fractures[7][8][9]. Postmenopausal OP was first associated with an excessive inflammatory reaction following the decrease of estrogens production in 1991. After only 2 weeks following oophorectomy, the authors have observed an increased urinary concentration of Interleukin-1 (IL-1) and Tumor Necrosis Factor-Alpha (TNF-α) compared with premenopausal women[10]. Interestingly, it was reported than an increased production of TNF-α and Receptor Activator of Nuclear factor Kappa-B Ligand (RANKL) in postmenopausal OP women was associated with an overactivation of T-cells responsible for an increased osteoclasts formation (Figure 1B)[11][12][13]. Leptin, an adipokine well known to be involved in food intake and energy metabolism, is also associated with bone metabolism. By fixing leptin receptor (Ob-R) on mesenchymal stem cells (MSCs), leptin stops adipocytic differentiation while it enhances osteoblastic differentiation and proliferation (Figure 1B)[14][15]. Surprisingly, the adipogenic differentiation of MSCs is higher in MSCs from postmenopausal women with OP compared with postmenopausal women without OP. While leptin is responsible for an antiadipogenic differentiation in postmenopausal women without OP, it had no effect on MSCs obtained from postmenopausal women with OP[16]. Interestingly, it is not only the secretion of cytokines and adipokines that seems to be dysregulated in OP. In bone microenvironment, Secreted Frizzled-Related Protein 1 (SFRP1), a Wnt canonical and non-canonical signaling pathway antagonist[17][18][19][20][21], is known to regulate the differentiation, proliferation and apoptosis of osteoblasts and osteocytes[22][23]. In fact, SFRP1 promotes MSCs differentiation in adipocytes and preadipocyte maturation, decreasing osteoblastogenesis (Figure 1B)[24][25][26]. Furthermore, by regulating the osteoblasts-induced osteoclastogenesis, SFRP1 is also involved in bone resorption process[27]. Interestingly, Tang et al. observed that SFRP1 and miR-144 serum levels were higher and positively correlated in postmenopausal osteoporotic women compared with postmenopausal women with normal bone density. They also reported that miR-144 promotes osteoblastic differentiation of bone marrow-derived MSCs by targeting SFRP1[28]. SFRP1 was also reported as down-regulated in the bone marrow of OP patients by Gu et al.[29]. In summary, we observe both a decrease of leptin-induced osteoblasts differentiation and an increased osteoblasts-induced osteoclastogenesis modulated by SFRP1. This suggests that the adipose tissue has a crucial role in bone metabolism and its dysregulation can promote metabolic disorders like OP. As reviewed by Kothari et al., adipose tissue is also a crucial player in breast tissue remodeling and carcinogenesis[30]. The relationship between osteoporosis and breast cancer is puzzling. Both diseases affect principally postmenopausal women after 65 years old. However, the biological explanation of such link remains misunderstood. OP risk decreases with estrogen exposure while breast cancer risk increases. Consequently, an older age at menarche and a younger age at menopause increase OP risk while they decrease breast cancer risk. On the other hand, weight under 55 kg at menopause increases OP risk, while obesity increases breast cancer risk in postmenopausal women[31]. A comparative study of both common and opposite biological and molecular aspects of both diseases could help to better manage women health.
3. Osteoarthritis
Osteoarthritis (OA) is a degenerative disorder of the joints induced by an increasing catabolic activity in both cartilage and bone tissues. OA is also described as a chronic wound due to initial cartilage injuries, inducing pro-inflammatory cytokines secretion in the synovial fluid and the associated immune system recruitment to repair injuries. Among the pro-inflammatory markers up-regulated in OA context, leptin, TNF-α, interleukin 6 (IL-6) and IL-1, known to negatively regulate cartilage formation[14][15][16][32][33]. IL-1 and TNF-α are produced by activated chondrocytes and synoviocytes in early OA and by leukocytes such as macrophages, T-cells and B-cells, which will then secrete many other pro-inflammatory cytokines including IL-6. Activated T helper 17 cells (Th17) increase interleukin 17 (IL-17) level in synovial fluid which is associated with an increased RANKL level, resulting in higher osteoclastogenesis (Figure 1B)[34][35]. It was reported that leptin controls not only bone formation but also bone resorption. By modulating RANKL expression, leptin decreases osteoclastic differentiation through the Beta-2 adrenergic receptor (ADRB2) expressed by osteoblasts[36]. SFRP1, which is also involved in osteoclastogenesis regulation is secreted by synovial cells and predominantly by fibroblasts-like cells of the synovial fluid in an OA context. However, Pasold et al. observed that in OA mouse models, SFRP1 expression is reduced in chondrocytes and MSCs, resulting in preferential osteoblastogenesis compared with chondrogenesis[37]. This increased osteoblastogenesis results in osteophytes production while the decreased chondrogenesis prevents cartilage healing resulting in a chronical inflammatory disorder. Once more, a subtle imbalance due to cartilage injuries in the joints results in MSCs preferential differentiation toward osteoblastic lineage compared with chondrocyte differentiation, which is needed to achieve cartilage healing. Interestingly, patients with knee or hip arthritis have a higher risk of breast cancer development after adjustment for age and sex[38]. However, adjustment for mammary gland history or stratification for histopathological characteristics of breast cancer lesions was unavailable. To date, references are insufficient to clearly understand the impact of OA on breast cancer risk.
4. Rheumatoid Arthritis
In contrast with OA which is initiated by cartilage lesions, rheumatoid arthritis (RA) is an autoimmune disease characterized by an uncontrolled immune reaction against both cartilage and bone tissue. RA development is associated with genetic predispositions and the presence of T-cell receptors at the joints[39][40]. Consequently, the recruitment of T-cells, notably T helper 1 (Th1) and Th17, results in IL-17 and TNF-α production, responsible for the increased production of IL-1 and IL-6 by macrophages and dendritic cells. This microenvironment promotes Th17 differentiation to the detriment of T regulators (Treg) differentiation. To complete the loop, IL-1, IL-6, IL-17, and TNF-α are known to stimulate osteoclastogenesis, which results in the degradation of mineralized tissue such as mineralized cartilage and subchondral bone[40][41][42]. Similar to what is observed in OA, leptin serum levels are higher in RA patients, so that overweight and obesity have been associated with RA[43][44]. MSCs were found in the synovium in a RA context, and, as described before, their differentiation is finely regulated by the microenvironment composition[45][46]. The administration of anti-Dikkopf-1 (Dkk-1) antibody in RA mouse models induces a decrease of bone erosion, potentially due to a decrease of osteoclast differentiation in the joint by decreasing levels of RANKL[47]. Lee et al. observed that the addition of SFRP1, another Wnt signaling antagonist in naïve T-cells medium is responsible for Th17 polarized T-cells differentiation. They also demonstrated that this differentiation is due to an increased sensitivity of T-cells to Transforming Growth Factor-Beta (TGF-ß)[48]. In murine models of arthritis, Matzelle et al. observed that the resolution of the inflammation resulted in a down-regulation of SFRP1 expression, a Wnt signaling antagonist. Consequently, by activating the Wnt signaling pathway, they also observed a decrease of bone resorption combined with an induction of osteoblast mineralization[49]. In breast, SFRP1 expression is higher during age-related lobular involution and in presence of microcalcifications compared with patients completely involuted and without microcalcification, respectively[50]. Interestingly, the incidence of RA in breast cancer patients is lower compared with patients without breast cancer after adjustment for age, comorbidities and breast cancer treatments[51]. However, the incidence of breast cancer in RA patients remains controversial. Bhandari et al. observed a higher cancer prevalence in RA patients, with a high proportion of breast cancer[52]. On the other hand, the meta-analysis of Tian et al. showed that the breast cancer risk in RA patient was not increased versus in the general population. However, when the population study was stratified for ethnicity, RA patients breast cancer risk was increased in non-Caucasian population while it decreased in the Caucasian population[53]. More recently, Wadström et al. observed a decreased occurrence of breast cancer in RA patients, also observable after adjustment for breast cancer treatment, suggesting that this reduction of breast cancer risk was already present before breast cancer treatment administration[54]. Unfortunately, the studied cohorts were not stratified for the presence of microcalcifications, the parity history or the degree of lobular involution, which could be a potential way of investigation to better understand the link between both diseases.
5. Periodontitis
This multibacterial-induced inflammatory disease is characterized by the destruction of periodontal tissues, a loss of alveolar bone mass principally due to an exacerbation of osteoclastogenesis, an inflammatory cells infiltration and an increased fibroblasts apoptosis. More precisely, after antigenic activation of T-cell surface glycoprotein CD4 positive (CD4+) naïve T-cells, activated Th1, T helper 2 (Th2), and Th17 produce cytokines responsible for the activation of B-cells, dendritic cells and neutrophils. Then, activated B-cells and T-cells produce RANKL responsible for an increased osteoclastogenesis[55][56][57][58][59][60]. Interestingly, Kawai et al. demonstrated that in healthy gingival tissue, only 20% of B-cells ant T-cells expressed RANKL. On the other hand, 50% of T-cells and 90% of B-cells expressed RANKL in a periodontitis (PD) context, which results in an abnormal alveolar bone destruction[57]. The clonal activation of B-cells induces the production of antibodies against gingival components such as collagen, resulting in the destruction of periodontal tissue. Numerous pro-inflammatory cytokines are upregulated in a periodontitis context including IL-1, IL-6, Interleukin-8 (IL-8), and TNF-α. The lack of Treg to control this inflammation completes the loop, and chronical inflammation then takes place in the periodontal tissue[55][56][57][58][59][60]. Leptin was also reported as upregulated in human saliva and circulating blood[61], and in dog periodontal ligament tissue[62] in a PD context. More recently, Zhu et al. performed a meta-analysis highlighting elevated leptin serum level and lower adiponectin serum level in PD patients compared with controls in the group with a body mass index under 30[63]. Li and Amar reported that anti-SFRP1 antibody was able to reverse both osteoclastogenesis and related inflammation, suggesting its crucial role in bone remodeling processes[64]. Surprisingly, multiple studies reported that PD is associated with breast cancer development, suggesting that this disorder could be a risk factor of breast cancer development[65][66][67]. However, no observation regarding a potential causal role of PD-related molecular issues on breast cancer development was reported yet. Investigations are still needed to conclude the existence of a link between PD and breast cancer development.
6. Other Osteoimmunological Disorders
The risk of developing an autoimmune rheumatic disease such as RA, systemic lupus erythematosus (SLE) or systemic sclerosis (SSc) in patients with breast cancer is lower compared with age and year of index date matched patients without breast cancer[51]. Reciprocally, the risk of developing breast cancer in SLE patients is lower compared with the general population[68][69][70][71][72][73]. On the other hand, Colaci et al. observed a higher incidence of breast cancer in SSc patients compared with age-sex-matched patients without SSc[74]. The same scheme is observable in psoriatic arthritis (PsA) patients. While some groups reported that breast cancer incidence was higher in PsA patients compared with age-sex-matched patients without PsA[75], others observed no difference in breast cancer occurrence between the two groups[76]. However, obesity and overweight incidence and prevalence in PsA patients are higher compared with the general population[77][78][79]. Obesity is a risk factor of triple-negative breast cancer development particularly in premenopausal women[80][81] and it is associated with poor breast cancer survival[82]. The higher breast cancer rate in the PsA group could be explained by a higher proportion of obese patients in the PsA group. Unfortunately, Wilton et al. did not report the body mass index (BMI) nor the breast cancer molecular subtype of the population studied. Divergent results could also potentially be explained by the absence of the cohort stratification regarding the degree of lobular involution, and the presence of microcalcifications. As described above, multiple studies reported a change in breast cancer prevalence or incidence in patients with osteoimmunological disorders, as well as few evidences of breast cancer effects on osteoimmunological disorders occurrence independently of breast cancer treatment. However, many adjustments for clinical variables as well as stratified analyses are lacking. Consequently, confounding variables are potentially responsible for multiple controversial results. Extensive studies are needed to conclude to the existence a potential link between osteoimmunological disorders and breast cancer incidence and this represent a new avenue of investigation to better personalize breast cancer treatment.
References
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536.osteoimmunological disorders;osteoimmunology;osteoclasts;osteoblasts;bone diseases;breast cancer;osteoporosis;rheumatoid arthritis;osteoarthritis;periodontitis
- Lorenzo, J. Cytokines and Bone: Osteoimmunology. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2020.format change
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245.
- Ralston, S.H.; Schett, G. Osteoimmunology. Calcif. Tissue Int. 2018, 102, 501–502.
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front. Immunol. 2020, 11, 58.
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642.
- Ettinger, B.; Pressman, A.; Sklarin, P.; Bauer, D.C.; Cauley, J.A.; Cummings, S.R. Associations between Low Levels of Serum Estradiol, Bone Density, and Fractures among Elderly Women: The Study of Osteoporotic Fractures. J. Clin. Endocrinol. Metab. 1998, 83, 2239–2243.
- Cummings, S.R.; Ensrud, K. Endogenous Hormones and the Risk of Hip and Vertebral Fractures among Older Women. N. Engl. J. Med. 1998, 339, 733–738.
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical Markers of Bone Turnover, Endogenous Hormones and the Risk of Fractures in Postmenopausal Women: The OFELY Study. J. Bone Miner. Res. 2000, 15, 1526–1536.
- Pacifici, R.; Brown, C.; Puscheck, E.; Friedrich, E.; Slatopolsky, E.; Maggio, D.; Mccracken, R.; Avioli, L.V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc. Natl. Acad. Sci. USA 1991, 88, 5134–5138.
- Breuil, V.; Ticchioni, M.; Testa, J.; Roux, C.H.; Ferrari, P.; Breittmayer, J.P.; Albert-Sabonnadière, C.; Durant, J.; De Perreti, F.; Bernard, A.; et al. Immune changes in post-menopausal osteoporosis: The Immunos study. Osteoporos. Int. 2010, 21, 805–814.
- D’Amelio, P.; Grimaldi, A.; Di Bella, S.; Brianza, S.Z.M.; Cristofaro, M.A.; Tamone, C.; Giribaldi, G.; Ulliers, D.; Pescarmona, G.P.; Isaia, G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone 2008, 43, 92–100.
- Adeel, S.; Singh, K.; Vydareny, K.H.; Kumari, M.; Shah, E.; Weitzmann, M.N.; Tangpricha, V. Bone Loss in Surgically Ovariectomized Premenopausal Women Is Associated With T Lymphocyte Activation and Thymic Hypertrophy. J. Investig. Med. 2013, 61, 1178–1183.
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003, 48, 3118–3129.
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50.
- Pablo Astudillo; Susana Ríos; Luis Pastenes; Ana Maria Pino; J. Pablo Rodriguez; Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. Journal of Cellular Biochemistry 2008, 103, 1054-1065, 10.1002/jcb.21516.
- Rehn, M.; Pihlajaniemi, T. Identification of three N-terminal ends of type XVIII collagen chains and tissue-specific differences in the expression of the corresponding transcripts. J. Biol. Chem. 1995, 270, 4705–4711.
- Bhanot, P.; Brink, M.; Samos, C.H.; Hsieh, J.C.; Wang, Y.; Macke, J.P.; Andrew, D.; Nathans, J.; Nusse, R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996, 382, 225–230.
- Finch, P.W.; He, X.; Kelley, M.J.; Uren, A.; Schaudies, R.P.; Popescu, N.C.; Rudikoff, S.; Aaronson, S.A.; Varmus, H.E.; Rubin, J.S. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. USA 1997, 94, 6770–6775.
- Bafico, A.; Gazit, A.; Pramila, T.; Finch, P.W.; Yaniv, A.; Aaronson, S.A. Interaction of Frizzled Related Protein (FRP) with Wnt Ligands and the Frizzled Receptor Suggests Alternative Mechanisms for FRP Inhibition of Wnt Signaling. J. Biol. Chem. 1999, 274, 16180–16187.
- Üren, A.; Reichsman, F.; Anest, V.; Taylor, W.G.; Muraiso, K.; Bottaro, D.P.; Cumberledge, S.; Rubin, J.S. Secreted frizzled-related protein-1 binds directly to wingless and is a biphasic modulator of wnt signaling. J. Biol. Chem. 2000, 275, 4374–4382.
- Bodine, P.V.N.; Zhao, W.; Kharode, Y.P.; Bex, F.J.; Lambert, A.-J.; Goad, M.B.; Gaur, T.; Stein, G.S.; Lian, J.B.; Komm, B.S. The Wnt Antagonist Secreted Frizzled-Related Protein-1 Is a Negative Regulator of Trabecular Bone Formation in Adult Mice. Mol. Endocrinol. 2004, 18, 1222–1237.
- Bodine, P.V.N.; Billiard, J.; Moran, R.A.; Ponce-de-Leon, H.; McLarney, S.; Mangine, A.; Scrimo, M.J.; Bhat, R.A.; Stauffer, B.; Green, J.; et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 2005, 96, 1212–1230.
- Boudin, E.; Fijalkowski, I.; Piters, E.; Van Hul, W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin. Arthritis Rheum. 2013, 43, 220–240.
- Monroe, D.G.; McGee-Lawrence, M.E.; Oursler, M.J.; Westendorf, J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene 2012, 492, 1–18.
- Taipaleenmäki, H.; Abdallah, B.M.; AlDahmash, A.; Säämänen, A.-M.; Kassem, M. Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp. Cell Res. 2011, 317, 745–756.
- Karl D Häusler; Nicole J. Horwood; Yoshiro Chuman; Jane L Fisher; Jennifer Ellis; T John Martin; Jeffrey S. Rubin; M.T Gillespie; Secreted Frizzled-Related Protein-1 Inhibits RANKL-Dependent Osteoclast Formation. Journal of Bone and Mineral Research 2004, 19, 1873-1881, 10.1359/jbmr.040807.
- Ling Tang; Wenjun Lu; Jian Huang; Xu Tang; Huiyun Zhang; Shujiao Liu; miR‑144 promotes the proliferation and differentiation of bone mesenchymal stem cells by downregulating the expression of SFRP1. Molecular Medicine Reports 2019, 20, 270-280, 10.3892/mmr.2019.10252.
- Huijie Gu; Liang Wu; Haihong Chen; Zhongyue Huang; Jun Xu; Kaifeng Zhou; Yiming Zhang; Jiong Chen; Jiangni Xia; Xiaofan Yin; et al. Identification of differentially expressed microRNAs in the bone marrow of osteoporosis patients.. American journal of translational research 2019, 11, 2940-2954.
- Charu Kothari; Caroline Diorio; Francine Durocher; The Importance of Breast Adipose Tissue in Breast Cancer. International Journal of Molecular Sciences 2020, 21, 5760, 10.3390/ijms21165760.
- T. Fontanges; Aurélie Fontana; Pierre Delmas; Osteoporosis and breast cancer.. Joint Bone Spine 2004, 71, 102-110, 10.1016/j.jbspin.2003.02.001.
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257.
- Michou, L.; Numan, M.; Amiable, N.; Brown, J.P. Paget’s disease of bone: An osteoimmunological disorder? DDDT 2015, 4695.
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 1–19.
- Chow, Y.Y.; Chin, K.-Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 1–19.
- Florent Elefteriou; Jong Deok Ahn; Shu Takeda; Michael Starbuck; Xiangli Yang; Xiuyun Liu; Hisataka Kondo; William G. Richards; Tony W. Bannon; Masaki Noda; et al.Karine ClementChristian VaisseGerard Karsenty Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514-520, 10.1038/nature03398.
- Juliane Pasold; Anja Osterberg; Kirsten Peters; Hanna Taipaleenmäki; Anna-Marja Säämänen; Brigitte Müller-Hilke; Brigitte Müller-Hilke; Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice. Experimental Cell Research 2013, 319, 649-659, 10.1016/j.yexcr.2012.12.012.
- Michael M Ward; Sara Alehashemi; Risks of solid cancers in elderly persons with osteoarthritis or ankylosing spondylitis. Rheumatology 2020, 59, 3817-3825, 10.1093/rheumatology/keaa166.
- Di Sante, G.; Tolusso, B.; Fedele, A.L.; Gremese, E.; Alivernini, S.; Nicolò, C.; Ria, F.; Ferraccioli, G. Collagen Specific T-Cell Repertoire and HLA-DR Alleles: Biomarkers of Active Refractory Rheumatoid Arthritis. EBioMedicine 2015, 2, 2037–2045. ]
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219.
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238.
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442.
- Taghadosi, M.; Samimi, Z.; Assar, S.; Salahshoor, M.R.; Jalili, C. Plasma Leptin Does Not Reflect the Effect of High Body Mass Index on Disease Activity in Rheumatoid Arthritis. Immunol. Investig. 2020, 49, 32–45.
- Rodríguez, J.; Lafaurie, G.I.; Bautista-Molano, W.; Chila-Moreno, L.; Bello-Gualtero, J.M.; Romero-Sánchez, C. Adipokines and periodontal markers as risk indicators of early rheumatoid arthritis: A cross-sectional study. Clin. Oral Investig. 2020.
- Jones, E.A.; English, A.; Henshaw, K.; Kinsey, S.E.; Markham, A.F.; Emery, P.; McGonagle, D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004, 50, 817–827.
- Djouad, F.; Bony, C.; Häupl, T.; Uzé, G.; Lahlou, N.; Louis-Plence, P.; Apparailly, F.; Canovas, F.; Rème, T.; Sany, J.; et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res. Ther. 2005, 7, R1304.
- Danielle Diarra; Marina Stolina; Karin Polzer; Jochen Zwerina; Michael S. Ominsky; Denise Dwyer; Adelheid Korb; Josef Smolen; Markus Hoffmann; Clemens Scheinecker; et al.Desiree Van Der HeideRobert LandeweDave LaceyWilliam G. RichardsGeorg Schett Dickkopf-1 is a master regulator of joint remodeling. Nature Medicine 2007, 13, 156-163, 10.1038/nm1538.
- Yoon-Sook Lee; Kyoo-A Lee; Hae-Bom Yoon; Seung-Ah Yoo; Young Woo Park; Yeonseok Chung; Wan-Uk Kim; Chang-Yuil Kang; The Wnt inhibitor secreted Frizzled-Related Protein 1 (sFRP1) promotes human Th17 differentiation. European Journal of Immunology 2012, 42, 2564-2573, 10.1002/eji.201242445.
- Melissa M. Matzelle; Maxime A. Gallant; Keith W. Condon; Nicole C. Walsh; Catherine A. Manning; Gary S. Stein; Jane B. Lian; David B. Burr; Ellen M. Gravallese; Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Care & Research 2012, 64, 1540-1550, 10.1002/art.33504.
- Alisson Clemenceau; Mirette Hanna; Kaoutar Ennour-Idrissi; Anna Burguin; Caroline Diorio; Francine Durocher; Secreted Frizzled-Related Protein 1 as a Biomarker against Incomplete Age-Related Lobular Involution and Microcalcifications’ Development. Cancers 2020, 12, 2693, 10.3390/cancers12092693.
- Hsin-Hua Chen; Ching-Heng Lin; Der-Yuan Chen; Wen-Cheng Chao; Yi-Hsing Chen; Wei-Ting Hung; Yin-Yi Chou; Yi-Da Wu; Chien-Chih Chen; Risk of major autoimmune diseases in female breast cancer patients: A nationwide, population-based cohort study. PLOS ONE 2019, 14, e0222860, 10.1371/journal.pone.0222860.
- Binita Bhandari; Bikash Basyal; Manbeer S. Sarao; Vinod Nookala; Yamin Thein; Prevalence of Cancer in Rheumatoid Arthritis: Epidemiological Study Based on the National Health and Nutrition Examination Survey (NHANES). Cureus 2020, 12, null, 10.7759/cureus.7870.
- Guo Tian; Jia-Ning Liang; Zhuo-Yun Wang; Dian Zhou; Breast Cancer Risk in Rheumatoid Arthritis: An Update Meta-Analysis. BioMed Research International 2014, 2014, 1-9, 10.1155/2014/453012.
- Hjalmar Wadström; Andreas Pettersson; Karin E Smedby; Johan Askling; Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Annals of the Rheumatic Diseases 2020, 79, 581-586, 10.1136/annrheumdis-2019-216756.
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk Between Bone and Immune System. Front. Endocrinol. 2019, 10, 236.
- Brunetti, G.; Colucci, S.; Pignataro, P.; Coricciati, M.; Mori, G.; Cirulli, N.; Zallone, A.; Grassi, F.R.; Grano, M. T Cells Support Osteoclastogenesis in an In Vitro Model Derived From Human Periodontitis Patients. J. Periodontol. 2005, 76, 1675–1680.
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.-P.; et al. B and T Lymphocytes Are the Primary Sources of RANKL in the Bone Resorptive Lesion of Periodontal Disease. Am. J. Pathol. 2006, 169, 987–998.
- Campbell, L.; Millhouse, E.; Malcolm, J.; Culshaw, S. T cells, teeth and tissue destruction—What do T cells do in periodontal disease? Mol. Oral Microbiol. 2016, 31, 445–456.
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease: Inflammatory and immune pathways in periodontal disease. Periodontology 2000 2014, 64, 57–80.
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1–14.
- Parth Purwar; M.A. Khan; Abbas Ali Mahdi; Shivani Pandey; Babita Singh; Jaya Dixit; Sagar Sareen; Salivary and Serum Leptin Concentrations in Patients With Chronic Periodontitis. Journal of Periodontology 2015, 86, 588-594, 10.1902/jop.2014.140581.
- Wei Li; Baoxin Huang; Kaining Liu; Jianxia Hou; Huanxin Meng; Upregulated Leptin in Periodontitis Promotes Inflammatory Cytokine Expression in Periodontal Ligament Cells. Journal of Periodontology 2015, 86, 917-926, 10.1902/jop.2015.150030.
- J. Zhu; Bin Guo; Xueqi Gan; Ling Zhang; Yuting He; Beilei Liu; Xin Chen; S. Zhang; Haiyang Yu; Association of circulating leptin and adiponectin with periodontitis: a systematic review and meta-analysis. BMC Oral Health 2017, 17, 104, 10.1186/s12903-017-0395-0.
- C.H. Li; Salomon Amar; Inhibition of SFRP1 reduces severity of periodontitis.. Journal of Dental Research 2007, 86, 873-877, 10.1177/154405910708600913.
- Shi, T.; Min, M.; Sun, C.; Zhang, Y.; Liang, M.; Sun, Y. Periodontal disease and susceptibility to breast cancer: A meta-analysis of observational studies. J. Clin Periodontol. 2018, 45, 1025–1033.
- Sfreddo, C.S.; Maier, J.; De David, S.C.; Susin, C.; Moreira, C.H.C. Periodontitis and breast cancer: A case-control study. Community Dent. Oral Epidemiol. 2017, 45, 545–551.
- Shao, J.; Wu, L.; Leng, W.-D.; Fang, C.; Zhu, Y.-J.; Jin, Y.-H.; Zeng, X.-T. Periodontal Disease and Breast Cancer: A Meta-Analysis of 1,73,162 Participants. Front. Oncol. 2018, 8, 601.
- Chan, K.; Clarke, A.E.; Ramsey-Goldman, R.; Foulkes, W.; Tessier Cloutier, B.; Urowitz, M.B.; Gladman, D.; Nived, O.; Romero-Diaz, J.; Petri, M.; et al. Breast cancer in systemic lupus erythematosus (SLE): Receptor status and treatment. Lupus 2018, 27, 120–123.
- Bernatsky, S.; Ramsey-Goldman, R.; Petri, M.; Urowitz, M.B.; Gladman, D.D.; Fortin, P.F.; Ginzler, E.; Romero-Diaz, J.; Peschken, C.; Jacobsen, S.; et al. Breast cancer in systemic lupus. Lupus 2017, 26, 311–315.
- Bernatsky, S.; Kale, M.; Ramsey-Goldman, R.; Gordon, C.; Clarke, A.E. Systemic lupus and malignancies. Curr. Opin. Rheumatol. 2012, 24, 177–181.
- Bernatsky, S.; Ramsey-Goldman, R.; Foulkes, W.D.; Gordon, C.; Clarke, A.E. Breast, ovarian, and endometrial malignancies in systemic lupus erythematosus: A meta-analysis. Br. J. Cancer 2011, 104, 1478–1481.
- Bernatsky, S.; Ramsey-Goldman, R.; Labrecque, J.; Joseph, L.; Boivin, J.-F.; Petri, M.; Zoma, A.; Manzi, S.; Urowitz, M.B.; Gladman, D.; et al. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J. Autoimmun. 2013, 42, 130–135.
- Parikh-Patel, A.; White, R.H.; Allen, M.; Cress, R. Cancer risk in a cohort of patients with systemic lupus erythematosus (SLE) in California. Cancer Causes Control 2008, 19, 887–894.
- Michele Colaci; Dilia Giuggioli; Caterina Vacchi; Federica Lumetti; Francesco Iachetta; Luigi Marcheselli; Massimo Federico; Clodoveo Ferri; Breast cancer in systemic sclerosis: Results of a cross-linkage of an Italian Rheumatologic Center and a population-based Cancer Registry and review of the literature. Autoimmunity Reviews 2014, 13, 132-137, 10.1016/j.autrev.2013.09.006.
- Katelynn M. Wilton; Cynthia S. Crowson; Eric L. Matteson; Malignancy incidence in patients with psoriatic arthritis: a comparison cohort-based incidence study. Clinical Rheumatology 2016, 35, 2603-2607, 10.1007/s10067-016-3396-5.
- Sherry Rohekar; Brian Tom; Agnes Hassa; Cathy T. Schentag; D. Farewell; Dafna D. Gladman; Prevalence of malignancy in psoriatic arthritis. Arthritis Care & Research 2007, 58, 82-87, 10.1002/art.23185.
- Bostoen, J.; Van Praet, L.; Brochez, L.; Mielants, H.; Lambert, J. A cross-sectional study on the prevalence of metabolic syndrome in psoriasis compared to psoriatic arthritis: Metabolic syndrome in psoriatic disease. J. Eur. Acad. Derm. Venereol. 2014, 28, 507–511.
- Haroon, M.; Gallagher, P.; Heffernan, E.; FitzGerald, O. High Prevalence of Metabolic Syndrome and of Insulin Resistance in Psoriatic Arthritis is Associated with the Severity of Underlying Disease. J. Rheumatol. 2014, 41, 1357–1365.
- Eder, L.; Harvey, P.; Chandran, V.; Rosen, C.F.; Dutz, J.; Elder, J.T.; Rahman, P.; Ritchlin, C.T.; Rohekar, S.; Hayday, R.; et al. Gaps in Diagnosis and Treatment of Cardiovascular Risk Factors in Patients with Psoriatic Disease: An International Multicenter Study. J. Rheumatol. 2018, 45, 378–384.
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Douglas Thompson, W.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597.
- Turkoz, F.P.; Solak, M.; Petekkaya, I.; Keskin, O.; Kertmen, N.; Sarici, F.; Arik, Z.; Babacan, T.; Ozisik, Y.; Altundag, K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013, 22, 344–350.
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635.
