Chimeric antigen receptors (CARs) are among the curative immunotherapeutic approaches that exploit the antigen specificity and cytotoxicity function of potent immune cells against cancers. Neuroblastomas, the most common extracranial pediatric solid tumors with diverse characteristics, could be a promising candidate for using CAR therapies. Several methods harness CAR-modified cells in neuroblastoma to increase therapeutic efficiency, although the assessment has been less successful. Regarding the improvement of CARs, various trials have been launched to overcome insufficient capacity. However, the reasons behind the inadequate response against neuroblastoma of CAR-modified cells are still not well understood. It is essential to update the present state of comprehension of CARs to improve the efficiency of CAR therapies.
Chimeric antigen receptors (CARs, also known as chimeric immunoreceptors, chimeric T cell receptors or artificial T cell receptors) are receptor proteins that have been engineered to give T cells the new ability to target a specific protein. The receptors are chimeric because they combine both antigen-binding and T-cell activating functions into a single receptor. CAR-T cell therapy uses T cells engineered with CARs for cancer therapy. The premise of CAR-T immunotherapy is to modify T cells to recognize cancer cells in order to more effectively target and destroy them. Scientists harvest T cells from people, genetically alter them, then infuse the resulting CAR-T cells into patients to attack their tumors. CAR-T cells can be either derived from T cells in a patient's own blood (autologous) or derived from the T cells of another healthy donor (allogenic). Once isolated from a person, these T cells are genetically engineered to express a specific CAR, which programs them to target an antigen that is present on the surface of tumors. For safety, CAR-T cells are engineered to be specific to an antigen expressed on a tumor that is not expressed on healthy cells. After CAR-T cells are infused into a patient, they act as a "living drug" against cancer cells. When they come in contact with their targeted antigen on a cell, CAR-T cells bind to it and become activated, then proceed to proliferate and become cytotoxic. CAR-T cells destroy cells through several mechanisms, including extensive stimulated cell proliferation, increasing the degree to which they are toxic to other living cells (cytotoxicity), and by causing the increased secretion of factors that can affect other cells such as cytokines, interleukins, and growth factors.- CAR T cells,immunotherapy,pediatric neuroblastom
1. Introduction
Neuroblastoma, an extracranial solid tumor that initiates from the sympathetic nervous system’s neuroendocrine tissue, is one of the most common causes of death in pediatric cancers [1][2]. It is often diagnosed during the perinatal period, which accounts for 8% in patients under 15 years. This childhood neoplasm appears each year in more than 600 cases in the United States and 200 in Japan [3][4][5]. According to clinical presentation, neuroblastoma is an extremely variant characteristic tumor. It ranges from an adrenal mass tumor that regresses without treatment to a metastatic tumor that causes critical illness [6]. At present, while intensive therapies can be beneficial for patients with localized disease, these therapies have frequently not been useful against patients with high-risk disease (approximately 40% of cases associated with the extent of metastases and genetic factors) nor patients with relapse [7][8]. Hence, novel therapies based on immunotherapy were subsequently developed to improve survival for high-risk patients.
One such approach is treatment with anti-GD2 monoclonal antibody, which has already been assessed in a Phase III clinical trial. The use of this antibody-based therapy was compiled into a therapeutic protocol for high-risk neuroblastoma patients and revealed promising results [9][10]. This effectiveness has led to other immunotherapeutic approaches, even though their integration into conventional multimodality therapies requires further investigation.
After discovering that GD2, a disialoganglioside highly expressed in most neuroblastomas, is also targeted by T cells, cellular immunotherapies including genetic engineering of T lymphocytes to express anti-GD2 chimeric antigen receptors (CARs) have emerged and are now being studied. With the combination of antigen specificity and cytolytic capacity, anti-GD2 CAR T cells have demonstrated safety and antitumor efficacy in relapsed neuroblastoma patients [11][12]. Various preclinical studies have improved the antitumor effects, proliferation, and cytokine release of CAR T cells, and some approaches have reached clinical trials. Currently, the anti-GD2 CAR T cell approach might represent a potential therapeutic for pediatric neuroblastoma.
2. CARs in Neuroblastoma
The knowledge of tumor immunology has been used in translating this comprehension into productive cancer therapies. A number of immunotherapy approaches represent a new borderline in treating cancers. One such strategy to overcome tolerance in cancer is to genetically engineer immune cells to express CAR. This concept of artificial antigen-specific receptors first originated in 1989–1993 [13][14]. By fusing an antibody-derived binding domain to T cell signaling domains, the CAR construct gains the tumor antigen specificity and the capacity to induce multiple signals in the response of immune cells. Over three decades, tremendous progress has been made and CARs were refined into the first, second, third, and currently fourth generations of their structure. Based on the potential of CAR T cells directed against the CD19 protein for treatment of hematologic malignancies shown in clinical trials, cancer immunotherapy was named the “Breakthrough of the Year” in 2013 by Science [15]. In addition, the use of anti-CD19 CAR T cells for relapsed/refractory acute lymphoblastic leukemia and in children and young adults was approved by the US Food and Drug Administration (FDA) in 2017 and two licensed products of CAR T cells including tisagenlecleucel and axicabtagene ciloleucel have been launched [16][17]. This success, therefore, has brought new insights for clinical translation in treating solid cancers.
CARs have been developed to fulfill the applicability of adoptive cellular immunotherapy for neuroblastoma in a major histocompatibility complex (MHC)-unrestricted manner in effector T cells. Effector immune cells, commonly T lymphocytes, have been genetically engineered to express an extracellular antigen-binding domain that is mostly a single-chain variable fragment (scFv) joined with a transmembrane domain and an intracellular signaling domain. The first-generation CARs were designed to have a single CD3-ζ intracellular signaling domain. The second- and third-generation CAR products were improved by adding one or two costimulatory endodomains to the CD3-ζ motif to achieve the optimal activation and survival of CAR cells. Current intracellular endodomains based on the costimulatory receptors include CD27, CD28, 41BB, ICOS, and OX40 [18][19]. Each of the CAR design components reflects the variations of therapeutics achievement, and novel CAR engineering has been developed for decades to broaden CAR therapeutics in solid tumors like neuroblastoma [20].
2.1. Summary of CAR Experience
Several CAR approaches in neuroblastoma have been developed according to discovered putative cancer antigens. There are some novel target antigens for CAR T cell therapy in neuroblastoma that have been investigated in the preclinical phase (Figure 1).

Figure 1. Target antigens conducted on the safety and efficacy of CAR therapy for neuroblastoma. Six surface antigens of neuroblastoma, including L1-CAM, GPC2, NCAM, GD2, ALK, and B7H3, are under development and investigation. L1-CAM and GD2 are the only two target antigens currently in completed clinical trials for neuroblastoma (labeled with star).
Anaplastic lymphoma kinase (ALK), an oncogene expressed in neuroblastoma cells, is associated with familial neuroblastoma cases [21][22]. Anti-ALK CAR has demonstrated its effectiveness against this neuroblastoma subtype in vitro and in vivo [23][24]. This line of research also suggested that antigen density must be considered to achieve CAR T cell potential. Another tyrosine kinase receptor that may be rendered an ideal target for CAR therapies is glypican 2 (GPC2). The high expression of GPC2 on the neuroblastoma cell surface brought promising clearance of disseminated neuroblastoma in the mouse model by anti-GPC2 CAR T cells [25]. B7H3 (CD276), a checkpoint molecule expressed in neuroblastomas, is another candidate for CAR therapies of neuroblastoma [26][27]. This attractive target brought useful immunotherapeutic strategies, including monoclonal antibodies and CARs targeting B7H3. Recently, the efficacy of anti-B7H3 CAR has been demonstrated in vivo [28][29]. Many target antigens that are specific to neuroblastoma cells have also been more characterized. Such antigens, including neural cell adhesion molecule (NCAM or CD56), New York esophageal squamous cell carcinoma 1 (NY-ESO1), and preferentially expressed antigen in melanoma (PRAME), were investigated both in vitro and in vivo for safety and efficacy, which gained attention for further development as CAR features [30][31][32][33].
To date, only CAR T cells targeting L1-CAM (CD171) and GD2 have reached the early phase of clinical trials. L1-CAM, an adhesion molecule in the immunoglobulin superfamily, is another suitable target in neuroblastoma [34]. Because of the specificity of CE7, the monoclonal antibody that can bind to the L1-CAM epitope, the anti-L1-CAM CAR with the scFv from CE7 was generated. The first-generation anti-L1-CAM CARs’ efficacy and safety were investigated in patients with relapsed/refractory neuroblastoma in a Phase 1 clinical trial [12]. To augment the persistence of anti-L1-CAM CAR, second-generation CAR was generated using a 41BB costimulation domain, followed by third-generation CAR, including CD28 costimulation addition, which is currently being investigated in phase 1 clinical trials [35][36]. Until now, the most critical target antigen in neuroblastoma has been GD2, a disialoganglioside highly expressed on neuroblastoma tissue [37]. Owing to the presence of this antigen during chemotherapy and the success of anti-GD2 monoclonal antibody therapy, this antigen has been the most studied targeted for CAR T cell therapy in neuroblastoma [38]. Many approaches of first-generation anti-GD2 CAR have been reported, including anti-GD2 CAR containing a single-chain variable fragment (scFv) derived from 14g2a monoclonal antibody or Epstein–Barr virus-specific cytotoxic T cell transduced CARs (so-called GD2 CAR-CTL), with the knowledge that the prolonged persistence in vivo was associated with the costimulation domain of CAR [39][40][41][42]. Anti-GD2 CAR constructs are now considered on costimulatory endodomains. The second and third generations of CAR were then generated for in vitro and in vivo assessments of CAR T cell survival [43][44]. The third-generation anti-GD2 CAR, containing an inducible caspase 9 (iC9) safety switch, has been tested in clinical trials for its safety (clinicaltrials.gov identifier NCT01953900 and NCT01822652). Various clinical trials based on CAR therapy are underway to augment the reliable therapeutic outcomes. However, improving the efficacy and persistence of CAR is still a significant issue.
2.2. Obstacles to Using CARs in Neuroblastoma
2.2.1. CAR T Cell Persistence and Exhaustion
Restrictive CAR T cell persistence has occurred as a major problem in neuroblastoma. Evidence from the first generation of CAR studies in vivo and clinical trials suggested that the limited persistence of CAR T cells from low activation and proliferation of cells also affected the antitumor efficacy [45][43][46][47]. One clinical study demonstrated that the infused, first-generation, anti-L1-CAM CAR cells were detectable in the peripheral blood up to 1–7 days after adoptive transfer in most patients with bulk disease but significantly longer (42 days) in a patient with limited disease burden [12]. T cell exhaustion might be a significant cause of shortening persistence. This is confirmed by discovering the exhausted CAR T cell phenotype in GD2 CAR T cells with low-level tonic signaling [48]. The persistence of infused CAR T cells might be prolonged if the exhaustion was reduced. Thus, several methods have been proposed to increase the persistence of CAR T cells. One such way is the utilization of second- and third-generation CARs, which improve costimulation after antigen binding (e.g., 4-1BB costimulatory domain) to protect shortened persistence. This development is under investigation for feasibility [49][50][48].
2.2.2. Target Selection and On-Target, Off-Tumor Effect
Ideally, the target antigen for CAR T cells should have a high expression in cancer cells, a low expression in normal cells, and not be associated with oncogenesis [51]. It is known that there are challenges in the path of choosing an optimal CAR T cell target antigen in neuroblastoma, since many target antigens are related to normal peripheral nerves or neural tissue expression. Toxicities caused by particular interactions between the CAR and its target antigen expressed by normal cells termed on-target, off-tumor effects have been reported in previous CAR studies in solid tumors [52][53][54][55]. One clinical trial in metastatic colon cancer reported pulmonary infiltration by CAR T cells that caused a systemic cytokine storm in patients who received HER2-targeted CAR T cell therapy, demonstrating strong evidence of on-target, off-tumor toxicities [54]. On the other hand, there was no such effect in a pediatric sarcoma study using anti-HER2 CAR T cells and anti-GD2 CAR T cells in neuroblastoma studies [42][56][57]. This evidence suggested that the variation of antigen density on the different types of cancer is an additional factor to consider during target selection to avoid on-target, off-tumor effects.
2.2.3. Tumor Microenvironment
Unlike the remarkable success of CAR T cells in the treatment of hematological malignancies, the efficacy of CAR T cells in neuroblastoma can be obstructed by the immunosuppressive tumor microenvironment (TME), which is a manifest barrier to achieve full effective CAR T cell therapy for solid tumors [58]. Significant factors derived from TME in neuroblastomas include immunosuppressive cells like tumor-associated macrophages (TAMs), Type 2 regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), which contributed to poor results of CAR therapy [59]. Another factor is the inhibitory ligands present in the TME, such as PD-L1, the ligand for an inhibitory receptor expressed on activated T cells, named PD-1 [60][61]. Remarkably, this habitual expression of the inhibitory ligand in neuroblastoma can cause the loss of CAR T cells [62]. In addition to inhibitory signals, the availability of soluble factors in the TME, including galactin 1 and 3, TGF-β, and IL-10, can trigger T cell inhibitory pathways or inhibit T cell function [61][63][64][65][66], while secretory HMGB1 may be responsible for Treg differentiation in the neuroblastoma TME [67]. Furthermore, there are physical barriers that prevent the tumor access of T cells, such as protease fibroblast activation protein (FAP) expressed by tumor-associated stromal fibroblasts, the extracellular matrix (ECM), and immunosuppressive tumor vasculature-like vascular endothelial growth factor (VEGF) [68][69].
2.2.4. CAR Trafficking
Trafficking of CAR cells into solid tumor sites to exert antitumor activity needs to be improved, especially in neuroblastoma. Several chemokines that can mediate immune cell trafficking are generally excreted by tumor or stromal cells like CC-chemokine ligand 17 (CCL17), CCL22, and CCL2 to enhance the localization of immune cells [70]. Moreover, suitable trafficking of immune cells, like T cells, can occur when there is an upregulation of a chemokine receptor that is matched to chemokine-related trafficking on T cells. However, in a previous study using CAR T cells derived from neuroblastoma patients, low expression of CCR2, a chemokine receptor, was detected [71][72][73]. Thus, various approaches to generate CAR T cells with an ability to traffic to neuroblastoma sites are underway.
1. Use in Cancer
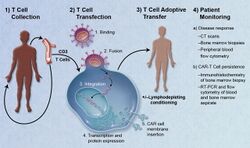
1. T-cells (represented by objects labeled as ’t’) are removed from the patient's blood.
2. Then in a lab setting the gene that encodes for the specific antigen receptors are incorporated into the T-cells.
3. Thus producing the CAR receptors (labeled as c) on the surface of the cells.
4. The newly modified T-cells are then further harvested and grown in the lab.
5. After a certain time period, the engineered T-cells are infused back into the patient.
Adoptive transfer of T cells expressing chimeric antigen receptors is a promising anti-cancer therapeutic as CAR-modified T cells can be engineered to target virtually any tumor associated antigen. There is great potential for this approach to improve patient-specific cancer therapy in a profound way. Following collection of a patient's T cells, the cells are genetically engineered to express CARs specifically directed toward antigens on the patient's tumor cells, then infused back into the patient.[6]
Preparation
The first step in the introduction of CAR-T cells into the body of a patient is the removal of activated leukocytes from the blood in a process known as leukocyte apheresis. The leukocytes are removed using a blood cell separator. The patient’s autologous peripheral blood mononuclear cells (PBMC) are then separated and collected from the buffy coat that forms.[7] The products of leukocyte apheresis are then transferred into a cell processing center. In the cell processing centre, specific T-cells are activated in a certain environment in which they can actively proliferate. The cells are activated using a type of cytokine called an interleukin, specifically Inter-Leukin 2 (IL-2) as well as anti-CD3 antibodies.[8] The T-cells are then transfected with CD19 CAR genes by either an integrating gammaretrovirus (RV) or by lentivirus (LV) vectors. These vectors are very safe in modern times due to a partial deletion of the U3 region.[9] The patient undergoes lymphodepletion chemotherapy prior to the introduction of the engineered CD CAR-T cells.[10] The depletion of the number of circulating leukocytes in the patient upregulates the number of cytokines that are produced which help to promote the expansion of the engineered CAR-T cell.[11]
Safety concerns
CAR-T cells are undoubtedly a major breakthrough in cancer treatment. However, there are still expected and unexpected toxicities that result from CAR-T cells being introduced into the body. These toxicities include cytokine release syndrome, neurological toxicity, on-target/off-tumor recognition, insertional mutagenesis, and anaphylaxis.[10] Cytokine release syndrome (CRS) is a condition in which the immune system is activated and releases an increased number of inflammatory cytokines. The clinical manifestations of this syndrome include high fever, fatigue, myalgia, nausea, tachycardia, capillary leakages, cardiac dysfunction, hepatic failure, and renal impairment.[12] The neurological toxicity associated with CAR-T cells have clinical manifestations that include delirium, the partial loss of the ability to speak a coherent language while still having the ability to interpret language (expressive aphasia), lowered alertness (obtundation), and seizures.[13] During some clinical trials deaths caused by neurotoxicity have occurred. The main cause of death from neurotoxicity is cerebral edema. In a study carried out by Juno Therapeutics, Inc., five patients enrolled in the trial died as a result of cerebral edema. Two of the patients were treated with cyclophosphamide alone and the remaining three were treated with a combination of cyclophosphamide and fludarabine.[14] In another clinical trial sponsored by the Fred Hutchinson Cancer Research Center, there was one reported case of irreversible and fatal neurological toxicity 122 days after the administration of CAR-T cells.[15] On-target/off-tumor recognition occurs when the CAR-T cell recognizes the correct antigen, but the antigen is expressed on healthy, non-pathogenic tissue. This results in the CAR-T cells attacking non-tumor tissue, such as healthy B cells that express CD19. The severity of this adverse effect can vary from B-cell aplasia to extreme toxicity leading to death.[8] Anaphylaxis is an expected side effect, as the CAR is made with a foreign monoclonal antibody and as a result, invokes an immune response. There is also a potential for insertional mutagenesis, which can occur when using a virus to insert the CAR vector DNA into a host T cell. Lentiviral (LV) vectors carry a lower risk than retroviral (RV) vectors. However, both have the potential to be oncogenic. Because it is a relatively new treatment, there is little data about the long-term effects of CAR-T cell therapy. There are still concerns about long-term survival as well as pregnancy complications in female patients treated with CAR-T cells.[13]
2. Structure
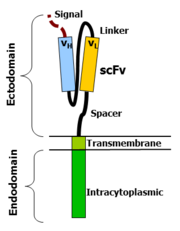
CAR-T cells create a link between an extracellular ligand recognition domain to an intracellular signalling molecule which in turn activates T cells. The extracellular ligand recognition domain is usually a single-chain variable fragment (scFv). CARs are composed of three regions: the ectodomain, the transmembrane domain and the endodomain.
Ectodomain
The ectodomain is the region of the receptor that is exposed to the extracellular fluid and consists of 3 components: a signalling peptide, an antigen recognition region and a spacer. A signal peptide directs the nascent protein into the endoplasmic reticulum. The signal protein in CAR is called a single-chain variable fragment (scFv),[16] a type of protein known as a fusion protein or chimeric protein. A fusion protein is a protein that is formed by merging two or more genes that code originally for different proteins but when they are translated in the cell, the translation produces one or more polypeptides with functional properties derived for each of the original genes.[17] A scFv is a chimeric protein made up of the light and heavy chains of immunoglobins connected with a short linker peptide. The linker consists of hydrophilic residues with stretches of glycine and serine in it for flexibility as well as stretches of glutamate and lysine for added solubility.[18]
Transmembrane domain
The transmembrane domain is a hydrophobic alpha helix that spans the membrane. The transmembrane domain is essential for the stability of the receptor as a whole. At present, the CD28 transmembrane domain is the most stable of the domains. Generally, the transmembrane domain from the most membrane proximal component of the endodomain is used. Using the CD3-zeta transmembrane domain may result in incorporation of the artificial TCR into the native TCR, a factor that is dependent on the presence of the native CD3-zeta transmembrane charged aspartic acid residue.[19] Different transmembrane domains result in different receptor stability. The CD28 transmembrane domain results in a highly expressed, stable receptor.
Endodomain
This is the functional end of the receptor. After antigen recognition, receptors cluster and a signal is transmitted to the cell.[16] The most commonly used endodomain component is CD3-zeta which contains 3 ITAMs. This transmits an activation signal to the T cell after the antigen is bound. CD3-zeta may not provide a fully competent activation signal and co-stimulatory signaling is needed. For example, chimeric CD28 and OX40 can be used with CD3-Zeta to transmit a proliferative/survival signal or all three can be used together.
3. History
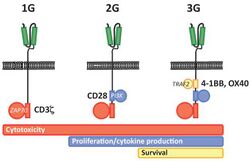
First generation CARs were developed in 1989 by Gideon Gross and Zelig Eshhar[21][22] at Weizmann Institute, Israel.[23] The first generation of CARs are composed of an extracellular binding domain, a hinge region, a transmembrane domain, and one or more intracellular signaling domains.[4] Extracellular binding domain contains single‐chain variable fragments (scFvs) derived from tumor antigen‐reactive antibodies and usually have high specificity to tumor antigen.[4] All CARs harbor the CD3ζ chain domain as the intracellular signaling domain, which is the primary transmitter of signals. Second generation CARs also contain co‐stimulatory domains, like CD28 and/or 4‐1BB. The involvement of these intracellular signaling domains improve T cell proliferation, cytokine secretion, resistance to apoptosis, and in vivo persistence.[4] Besides co-stimulatory domains, the third‐generation CARs combine multiple signaling domains, such as CD3z-CD28-41BB or CD3z-CD28-OX40, to augment T cell activity. Preclinical data shows the third-generation CARs exhibit improved effector functions and in vivo persistence as compared to second‐generation CARs.[4] Recently, the fourth‐generation CARs (also known as TRUCKs or armored CARs), combine the expression of a second‐generation CAR with factors that enhance anti‐tumoral activity (e.g., cytokines, co‐stimulatory ligands).[24] The evolution of CAR therapy is an excellent example of the application of basic research to the clinic. The PI3K binding site used was identified in co-receptor CD28,[25] while the ITAM motifs were identified as a target of the CD4- and CD8-p56lck complexes.[26] The introduction of Strep-tag II sequence (an eight-residue minimal peptide sequence (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) that exhibits intrinsic affinity toward streptavidin[27]) into specific sites in synthetic CARs or natural T-cell receptors provides engineered T cells with an identification marker for rapid purification, a method for tailoring spacer length of chimeric receptors for optimal function and a functional element for selective antibody-coated, microbead-driven, large-scale expansion.[28][29] Strep-tag can be used to stimulate the engineered cells, causing them to grow rapidly. Using an antibody that binds the Strep-tag, the engineered cells can be expanded by 200-fold. Unlike existing methods this technology stimulates only cancer-specific T cells.
Smart T cell
Combined with exogenous molecules, some synthetic control devices have been implemented on CAR-T cells and alter the cell activity. Smart T cell is engineered with suicide gene or other synthetic control panels to precisely control therapeutic function over the timing and dosage, there by alleviating cytotoxicity.[30] Several strategies to improve safety and efficacy of CAR-T cells are: Suicide gene engineering: engineered T cells are incorporated with suicide genes, which can be activated by extracellular molecule and then induce T cell apoptosis. Herpes simplex virus thymidine kinase (HSV-TK) and inducible caspase 9 (iCas9) are two types suicide genes have been integrated into CAR-T cells.[30][31][32] In iCas9 system, the suicide gene is composed of the sequence of the mutated FK506-binding protein with high specificity to a small-molecule, AP1903 and a gene encoding human caspase 9 switch. When the release of cytokines by CAR-T cells becomes more pronounced than basic levels, the iCas9 can be dimerized and lead to rapid apoptosis of T cells. Although both suicide genes demonstrate a noticeable function of as a safety switch in clinical trials for cellular therapies, some hinder defects limit the application of this strategy. HSV-TK is derived from virus and may be immunogenic to humans.[30][33] The suicide gene strategies may not act quickly enough to eliminate off-tumor cytotoxicity as well. Dual-antigen receptor: T cells are engineered to express two tumor-associated antigen receptors at the same time. The dual-antigen receptor of engineered T cell module has been reported to have less intense side effects.[34] The activation of CAR-T cell via TCR-CD3ζ signal transduction pathway is transient and a complementary signal pathway provided by co-stimulatory molecules on antigen presenting cells promotes survival of modified-T cell can ability in controlling tumor.[35] An in vivo study in mice shows the dual-receptor T cells effectively eradicated prostate cancer and achieved complete long-term survival.[36] ON-switch: ON-switch CAR-T cell split synthetic receptors into two parts: the first part mainly contains an antigen binding domain towards and the other part features two different downstream signaling elements (e.g. CD3ζ and 4-1BB). Upon the presence of an exogenous molecule (rapamycin analogs for example), two physically separated signaling elements fuse together and CAR-T cells exert therapeutic functions.[37] In this mechanism, the engineered T cell shows therapeutic function only in the presence of both tumor antigen and a benign exogenous molecule. Bifunctional molecules as switches: The bispecific antibodies are developed as an efficacious bridge to target cytotoxic T cells to cancer cells and causes localized T cell activation. In this strategy, the bispecific antibody targets CD3 molecule of T cell and tumor-associated antigen presented on cancer cell surface.[38] The anti-CD20/CD3 bispecific molecule shows high specificity to both malignant B cells and cancer cells in mice.[39] FITC is another bifunctional molecule used in this strategy. FITC can redirect and regulate the activity of the FITC-specific CAR-T cells toward tumor cells with folate receptors.[40]
SMDC adaptor technology
SMDCs (small molecule drug conjugates) platform in immuno-oncology is a novel (currently experimental) approach that makes possible the engineering of a single universal CAR T cell, which binds with extraordinarily high affinity to a benign molecule designated as FITC. These cells are then used to treat various cancer types when co-administered with bispecific SMDC adaptor molecules. These unique bispecific adaptors are constructed with a FITC molecule and a tumor-homing molecule to precisely bridge the universal CAR T cell with the cancer cells, which causes localized T cell activation. Anti-tumor activity in mice is induced only when both the universal CAR T cells plus the correct antigen-specific adaptor molecules are present. Anti-tumor activity and toxicity can be controlled by adjusting the administered adaptor molecule dosing. Treatment of antigenically heterogeneous tumors can be achieved by administration of a mixture of the desired antigen-specific adaptors. Thus, several challenges of current CAR T cell therapies, such as:
- the inability to control the rate of cytokine release and tumor lysis
- the absence of an “off switch” that can terminate cytotoxic activity when tumor eradication is complete
- a requirement to generate a different CAR T cell for each unique tumor antigen
may be solved or mitigated using this approach.[41][42][43]
4. Clinical Studies
As of August 2017, there were around 200 clinical trials happening globally involving CAR-T cells.[10] Around 65% of those trials targeted blood cancers, and 80% of them involved CD19 CAR-T cells targeting B-cell cancers.[10] In 2016, studies began to explore the viability of other antigens, such as CD20.[44]
FDA approvals
The first two FDA approved CAR-T therapies both target the CD19 antigen, which is found on many types of B-cell cancers.[45] Tisagenlecleucel (Kymriah) is approved to treat relapsed/refractory B-cell precursor acute lymphoblastic leukemia (ALL), while axicabtagene ciloleucel (Yescarta) is approved to treat relapsed/refractory diffuse large B-cell lymphoma (DLBCL).[45] Kymriah's clinical trial showed an 83% remission rate of all types of B-cell ALL after three months post treatment.[46] However, 49% of patients also suffered cytokine release syndrome (CRS), a serious side effect that has been responsible for several deaths in clinical trials run by Novartis’ competitors.[46]