2. Quercetin Particularities
2.1. Sources
Quercetin is the most widely distributed flavonoid in the world; it is usually found as a component in certain types of plants such as capers, tea, tomatoes, garlic, spinach, and brucella cabbage; in fruits such as grapes, apples, berries, pears, and cherries; and in seeds, nuts, flowers, bark, and leaves. In addition, it is also found in certain plants of medicinal use such as
Hypericum perforatum (St. John’s wort) and
Sambucus canadensis (elderberry). Most of the quercetin consumed daily in the diet is in the glycosidic form, which is formed by the addition of a glycosyl group (a sugar such as glucose, rhamnose, or rutin) instead of an -OH group (position 3), while the aglycosidic form is found in much smaller amounts. One of the significant sources of quercetin aglycone is the onion; quercetin aglycone is more abundant in an onion’s outermost layers, while the innermost layers contain more substantial quantities of quercetin glycosides. Up to 10% of an onion’s dry weight can comprise quercetin in various forms
[5]. In addition, the polyphenolic profile of fruits and vegetables depends on the plant species, growing conditions, harvesting conditions, and storage methods. For example, storage at high or very low temperatures reduces the quercetin content in foods. Price, in 1997, evaluated the curing process of onions at 28 °C for six months, during which he observed a decrease in quercetin content between 50 and 60%, while when immersed in water at 100 °C, only 25–33% of their content decreased. In contrast, the quercetin content in strawberries stored at 20 °C for nine months showed an increase of about 32%
[9][10][9,10].
2.2. Physicochemical Properties
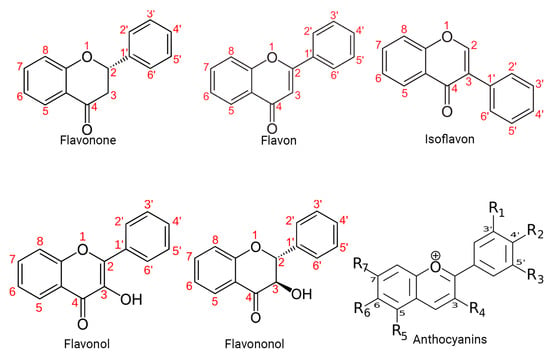
Specifically, polyphenols can be broadly classified into flavonoids and nonflavonoids. On the other hand, the structural diversity of flavonoid molecules arises from variations in the hydroxylation pattern and oxidation state of the central pyran ring, resulting in a broad spectrum of compounds, including flavanols, anthocyanidins, anthocyanins, isoflavones, flavones, flavonols, flavanones, and flavanonols (some of these structures are depicted in
Figure 1)
[11].
Figure 1. Structural diversity of flavonoids. This figure presents a visual representation of the structural diversity of flavonoid molecules. Variations in the hydroxylation pattern and oxidation state of the central pyran ring lead to the formation of a wide spectrum of compounds.
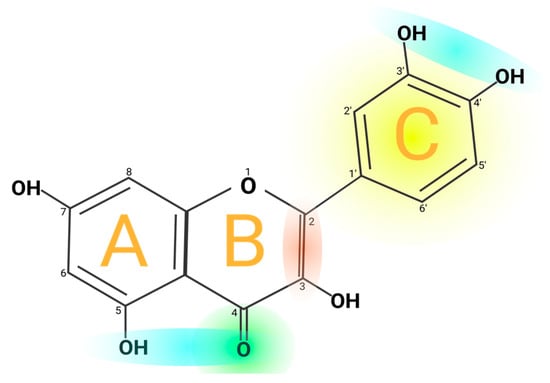
Quercetin’s structure is 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one with a molecular weight of 302.24 g/mol and a melting point of 316 °C, and its molecular formula is C
15H
10O
7. Quercetin consists of five hydroxyl groups (
Figure 2), which determine the biological activity of the compound
[2][12][2,12]. Naturally, quercetin is distributed in the form of derivatives, either in glycosidic form, mainly attached to the C-3 carbon with glucose, rhamnose, and rutinose, or linked to others, and it is very rarely distributed as an aglycone
[13]. On the other hand, quercetin in its aglycone form is a bright lemon-yellow powder, insoluble in water but soluble in alcohol and lipids. In addition, the aglycone has been reported to have a minor effect in vivo and poor bioavailability in human plasma after oral intake of quercetin
[14][15][14,15].
Figure 2. Chemical structure of quercetin. The figure shows the chemical characteristics that give it its antioxidant capacity, the most important of which is the catechol or dehydroxylated B-ring structure (yellow). Other important characteristics are the presence of unsaturation in the C-ring (red) and the presence of a 4-oxo in the C-ring (green); the oxygen in position 4 and the hydroxyl in position 5 allow it the capacity to chelate ions such as Cu++ and Fe++ (blue). On the other hand, in total, quercetin has 5 functional hydroxyl groups with the potential to be conjugated and that differ in their chemical reactivity following the order of reactivity 3 > 7 > 3′ > 4′ >> 5. The phenolic hydroxyl groups of quercetin act as donors of electrons, and these are responsible for the capture activity of free radicals, in particular the catechol structure with 2 hydroxyl groups in the neighboring positions, which is notably superior to other arrangements in electron donation.
However, quercetin is highly lipophilic in nature due to the presence of five hydroxyl groups. However, the solubility of quercetin derivatives depends on the type of substituent molecules present in the OH group. The O-methyl, C-methyl, and prenyl derivatives of quercetin are lipophilic and are widely found on the surface of leaves, flowers, and fruits of the Labiatae or Compositae families
[16]. Nevertheless, the glycosylation of quercetin increases its hydrophilic properties, and these glycosylated derivatives are soluble in the cytosol of plants, are easily transported to all parts of plants, and are mainly stored in the vacuoles
[4]. In addition, the unique chemical structure of quercetin is responsible for its potent antioxidant properties. The structural groups responsible for quercetin’s stability and antioxidant activity are the ortho-dihydroxy or catechol group and the 3- and 5-OH groups in conjugation with the 4-oxo group
[17]. Therefore, quercetin donates a proton to free radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH) and is converted to quinone intermediates that are stabilized by the electrons donated by these functional groups. Quercetin derivatives, such as C3 and C4OH glycoside derivatives, have a lower H
+ donating capacity. In addition, the reducing potential of the C3OH derivatives of quercetin is higher compared to its aglycone form
[18][19][18,19].
2.3. Bioavailability and Pharmacokinetics
Bioavailability is the fraction of an ingested substance absorbed and available at the place of action. It is known that the bioavailability of quercetin is usually relatively low (0.17–7 μg/mL), less than 10% of what is consumed, due to its poor water solubility (hydrophobicity), chemical stability, and absorption profile. The bioavailability of quercetin depends on its chemical structure, origin, physicochemical properties, and whether it is consumed in parallel with other compounds, e.g., fats and pectin. In addition, a characteristic feature of the bioavailability of these metabolites is their slow elimination since their half-life ranges from 11 to 48 h, which could favor their accumulation in plasma after repeated intakes
[20]. Several studies suggest that glycosidic quercetin is metabolized in the gastrointestinal tissue and liver before it enters the blood and is distributed to the internal organs. The acidic environment in the stomach does not facilitate the absorption of quercetin bound to sugars. However, this process is mediated by gastrointestinal enzymes such as phlorizin lactase hydrolase and cytosolic β-glucosidases in the colon, microbial β-glucosidases, and α-rhamnosidase
[21].
Furthermore, quercetin cannot be absorbed by a passive mechanism because it is too polar to cross the phospholipid bilayers in the cell walls of the epithelium. However, thanks to metabolic enzymes secreted by the human body and the intestinal microbiota, quercetin undergoes chemical transformations that allow it to be absorbed in the gastrointestinal tract
[22]. Concerning quercetin aglycone, which is highly lipophilic, it is proposed that it could cross the enterocyte membrane by simple diffusion
[4]. On the other hand, in tissue, quercetin and quercetin glycosides from foods appear conjugated, either glucuronidated, sulfated, or methylated, suggesting that the in vivo bioactivity of quercetin may be due to its metabolites
[21]. Quercetin glycosides can be absorbed by an active transport mechanism due to sodium-dependent glucose transporter 1 (SLGT1), a protein embedded in the cell walls of the gastrointestinal epithelium. Ingested quercetin glycosides can also be absorbed due to the activity of lactase-phlorizin hydrolase (LPH), an enzyme that removes the sugar moiety, resulting in a more hydrophobic quercetin aglycone. After absorption, the aglycone form can passively diffuse into the hepatic portal vein and then be transported throughout the body. In the intestine, biotransformation of quercetin results in the production of methyl metabolites, sulfate, and glucuronide, which tend to be more readily absorbed and can be measured in urine and blood to determine bioavailability
[22][23][22,23].
To improve their bioavailability, several formulation strategies have been developed, such as various encapsulation techniques, in which an active ingredient is enclosed in another material to enhance its handling, dispersibility, stability, and/or functionality, including nanoencapsulation; emulsification; the use of liposomes or hydrogels; and complexation with other molecules such as cyclodextrin and cocrystallization. However, studies that promote the achievement of high bioavailability, whether in vivo or ideally in clinical intervention studies, still need to be strengthened
[23]. Most published studies examined quercetin and/or its metabolites in the urine and plasma of relatively small numbers of volunteers, where there is less variation in metabolites derived from absorption in the small intestine compared to catabolites derived from the action of the colonic microbiota. Thus, dietary history, genetic polymorphisms, and variations in intestinal microbiota metabolism have been found to play an essential role in the bioavailability of quercetin
[24].
2.4. Human Absorption and Metabolism of Quercetin
As mentioned, quercetin is in the form of glycosides in vegetables and fruits. The degree of absorption of quercetin is given by the glycosides’ nature and binding site at positions 3, 5, 7, or 4
[25][26][25,26]. For example, Ullah H. et al. determined the absorption of various forms of quercetin in human volunteers using healthy subjects with ileostomy to avoid bacterial degradation in the colon. Their results showed that quercetin absorption was 52% for an onion-rich meal, 24% for quercetin supplementation, and 17% for routine supplementation
[25][27][28][25,27,28]. Once ingested, quercetin glycosides are rapidly hydrolyzed by the enzyme β-glucosidase in the epithelial cells of the upper small intestine, much of which is subsequently absorbed.
Similarly, the glucuronic acid in quercetin and its sulfuric acid derivatives are more readily absorbed than quercetin. However, it has not been reported whether quercetin and its derivatives are stable in gastric acid; therefore, there are no reports on whether they can be absorbed in the stomach
[23]. In addition, purified quercetin glycosides can interact with sodium-dependent glucose transport receptors in the mucosal epithelium and, therefore, can be absorbed by the small intestine in vivo
[29].
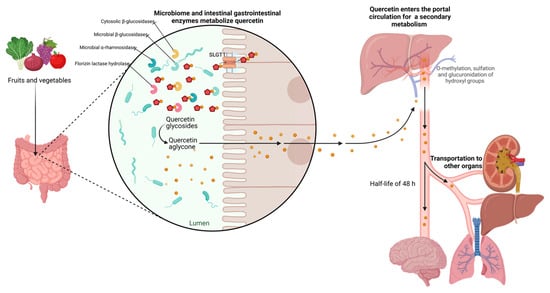
However, rutin and other quercetin glycosides bound to oligosaccharides or polysaccharides are absorbed in the lower part of the digestive tract (large intestine) in the form of aglycones through deglycosylation by enterobacteria (
Eubacterium ramulus,
Clostridium orbiscindens,
Eubacterium oxidoreducens,
Butyrovibrio spp.). In contrast, quercetin monoglucosides such as isoquercitrin and quercetin-4’-glucoside (Qu4’G) are absorbed in the upper part of the intestine (small intestine) after enzymatic hydrolysis by β-glucosidase and/or lactase-phlorizin hydrolase (LPH) in the intestinal mucosa derived from intestinal microbiota, as shown in
Figure 3. Some of the quercetin monoglucosides can be taken up by sodium-glucose cotransporter 1 (SGLT-1). These metabolites enter the gastrointestinal tract via multidrug resistance-associated protein 2 (MRP2) and are subsequently transported through the blood vessels to the liver, where they are subjected to secondary metabolism
[30]. After absorption, quercetin binds to albumin and is transported primarily to the liver via the portal vein and to other organs, including the small intestine, colon, and kidney. In the liver, it undergoes O-methylation, glucuronidation, and/or sulfation to form its conjugates quercetin-3-glucuronide, quercetin-30-sulfate, and isorhamnetin-3-glucuronide
[31]. Conjugation reactions with glucuronic acid and/or sulfate appear to be the most common type of flavonoid metabolic pathway. For example, glucuronidation of flavanols occurs in human microsomes and the liver, and uridine diphosphate (UDP)- glucuronosyltransferase 1A9 (UGT-1A9) plays a vital role in this process. Kuhnle et al. reported that glucuronidation, O-methylation, and O-methyl-glucuronidation are part of flavonoid metabolism in the small intestine
[32][33][32,33].
Figure 3. Metabolism of quercetin. The diagram illustrates the enzymatic transformations undergone by quercetin within the biological system. Key steps include deglycosylation by enterobacteria facilitating its absorption, and hydroxylation, glycosylation, and methylation processes, leading to the formation of various metabolites in the liver. The figure highlights the network of metabolic reactions involved in the biotransformation of quercetin, shedding light on its fate within the body.
2.5. Quercetin Excretion
Flavonoid glucuronides and sulfates are polar, water-soluble compounds that mammals efficiently excrete in urine and bile. When discharged in the bile, flavonoids pass into the duodenum and are metabolized by intestinal bacteria, producing cleavage products and/or the hydrolysis of glucoronoconjugates or sulfoconjugates
[33]. The released metabolites can be reabsorbed and enter an enterohepatic cycle. The substitution in the flavonoid molecule, the degree of polarity, and the molecular weight determine the degree of biliary excretion
[34]. It is worth mentioning that flavonoids are also eliminated through renal excretion in the liver after conjugation. Studies have demonstrated that the amount of excreted flavanols, as a proportion of intake, can vary from 0.8% to 1.4% depending on the dietary source of flavanols. The elimination half-life can also be influenced, with plasma levels of quercetin detected up to 48 h after consuming flavanols
[5].
2.6. Pharmacological Properties
In addition to its antioxidant capacity, different pharmacological properties associated with the administration of quercetin have been described, such as its carcinostatic properties (antimutagenic properties, suppression of cell proliferation, and induction of apoptosis), antiviral properties (against human immunodeficiency virus (HIV), hepatitis B virus (HBV), and herpes simplex virus (HSV)), antihypertensive properties (decrease in ventricular hypertrophy), anti-inflammatory properties, protection of low-density lipoprotein (LDL) from oxidation, and inhibition of angiogenesis
[35]. Some of the main pharmacological properties of quercetin are described in more detail below.
2.6.1. Antioxidant Properties
Oxidative stress refers to the pathophysiological responses caused by the excessive production of highly reactive molecules such as reactive oxygen species (ROS) in the body and the dysregulation of the oxidative–antioxidative balance when subjected to various harmful stimuli. Oxidative stress can damage mitochondrial DNA, denature intracellular proteins, cause lipid peroxidation, and drive inflammation
[36]. Due to the phenolic hydroxyl group and a double bond, quercetin exhibits potential antioxidant activity. Quercetin is a potent scavenger of reactive oxygen species (ROS), protecting the organism against oxidative stress. Moreover, quercetin maintains oxidative balance, making it a robust antioxidant, and regulates the level of glutathione (GSH) in the body. Animal and cell studies have demonstrated that quercetin induces the synthesis of GSH. For instance, Xu and Dong described an increase in the expression of superoxide dismutase (SOD), catalase (CAT), and GSH with quercetin pretreatment
[37]. In this regard, quercetin enhances the antioxidant capacity of the organism by elevating levels of glutathione (GSH). This is attributed to the fact that once oxygen free radicals are generated in the body, superoxide dismutase (SOD) rapidly captures O
2− and converts it into H
2O
2. Additionally, this enzyme catalyzes the breakdown of H
2O
2 into nontoxic H
2O. This reaction necessitates GSH as a hydrogen donor
[38].
In addition to its direct antioxidant properties inherent to the chemical family to which it belongs, quercetin can interact with the endogenous antioxidant network by activating the antioxidant responsive element (ARE). The ARE is a consensus sequence responsible for starting the transcription of most endogenous antioxidant enzymes, including notable ones such as NADPH dehydrogenase quinone 1 (NQO1), glutathione S-transferase (GSTA), thioredoxin (TRX), heme oxygenase (HMOX1), ferritin light chain (FTL), and inducible nitric oxide synthase (NOS). The mechanism by which quercetin accomplishes this process involves increasing the activity of the nuclear factor erythroid 2-related factor 2 (NRF2), enhancing its binding to the ARE, reducing its degradation, and consequently augmenting the synthesis of the involved enzymes
[39].
2.6.2. Cardiovascular Disease
Hypertensive Activity
High blood pressure is a condition in which the blood pressure in the arteries is constantly high. This condition can be dangerous because it can increase the risk of cardiovascular disease and stroke. Quercetin has been shown to benefit hypertension by lowering blood pressure in animal and human studies
[40]. For example, Abdelghffar et al. found a decrease in systolic, diastolic, and mean blood pressure, a reduction in ventricular hypertrophy, and less damage to the renal and vascular parenchyma in a model of hypertensive rats. These findings are due in part to the vasodilator property of quercetin, which in turn is explained by quercetin’s ability to scavenge free radicals, which would usually activate the secretion of 8-iso-prostaglandin F2α, a potent vasoconstrictor hormone
[41]. Likewise, a study published in the British Journal of Nutrition found that daily supplementation with quercetin (730 mg) for 12 weeks significantly reduced blood pressure in obese and overweight adults with prehypertension and stage 1 hypertension
[42]. In addition, some underlying molecular mechanisms by which quercetin may be acting have been investigated. One theory is that quercetin causes endothelial nitric oxide synthase (eNOS) to be activated, producing nitric oxide (NO), a vasodilator that relaxes blood vessels and reduces blood pressure. According to several studies, quercetin increases eNOS activity and NO generation
[43]. Another mechanism by which quercetin may exert its antihypertensive effect is the inhibition of angiotensin converting enzyme (ACE). Angiotensin II, a potent vasoconstrictor that raises blood pressure, is created by the ACE enzyme, and quercetin has been shown to inhibit ACE activity, reducing blood pressure
[44].
Cardiovascular Protection
Several studies have shown that quercetin has positive effects on cardiovascular diseases. Suri et al. evaluated the impact on vasoconstrictor and vasodilator reactions in the porcine heart, reporting that quercetin increased both cyclic guanosine mono-phosphate (cGMP) content and cGMP-dependent relaxations to glyceryl trinitrate (GTN) and sodium nitroprusside (SNP), as well as porcine receptor-mediated restrained contractions
[45]. Also, a study with rats showed that quercetin (0.1–100 μM) relaxed the contraction induced by pretreatment with five mM norepinephrine in a concentration-dependent manner. It was concluded that quercetin induces Ca
++ elevation, leading to NO production and activation of the endothelial cell’s Ca
++-activated K
+ channel (KCa)
[46]. It should also be mentioned that quercetin showed anti-inflammatory properties in patients with coronary artery disease (CAD) through a decrease in nuclear factor kappa B (NF-Κb) transcriptional activity. Chekalina et al. determined the effect of quercetin in patients with CAD to test this effect. Eighty-five patients with CAD participated in the study. Thirty patients received quercetin at a daily dose of 120 mg for two months, while the remaining fifty-five patients considered as a control group received β-blockers, statins, and aspirin. Increased levels of interleukin 1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-10 were detected in the serum of CAD patients. Under the influence of quercetin, the levels of interleukin 10 (IL-10), IL-1β, and TNF-α were reduced. Also, quercetin decreased the expression of the βα kappa inhibitor (Ikβα) relative to the control group
[47].
2.6.3. Alzheimer’s Disease
It has also been proposed that quercetin may have therapeutic effects on Alzheimer’s disease (AD) through several molecular mechanisms. Oxidative stress and neuroinflammation play a crucial role in the pathogenesis of AD, and quercetin can scavenge free radicals and inhibit the production of inflammatory cytokines, thereby reducing oxidative stress and inflammation in the brain
[48]. Another mechanism is quercetin’s ability to modulate the enzyme activity in clearing amyloid-beta (Aβ) plaques, a hallmark of AD pathology. Quercetin can increase the activity of neprilysin and insulin-degrading enzymes responsible for degrading amyloid beta (Aβ) plaques in the brain, thereby reducing their accumulation and toxicity
[49]. Quercetin can also modulate the activity of kinases involved in tau protein phosphorylation, another hallmark of AD pathology.
Furthermore, quercetin can inhibit the activity of glycogen synthase kinase 3β, responsible for abnormal tau protein phosphorylation, thus reducing tau aggregation and neurofibrillary tangles in the brain
[49]. Many in vivo studies demonstrated that the hydroxy-functional groups in the B-ring of quercetin are essential in inhibiting Aβ aggregation and altering mature fibrils by forming hydrogen bonds with the β-sheet structure. In addition, other studies have shown that quercetin increased the survival of neuronal cultures in vivo. Likewise, quercetin at 100 μM showed considerable inhibition of beta-site amyloid precursor protein cleaving enzyme 1 (BACE1) in an in vivo system by 11.85%
[50]. The other characteristic pathological implication of AD is the appearance of neurofibrillary tangles (NFTs), with tau being the central protein. Several studies demonstrated that quercetin effectively inhibited tau accumulation through different mechanisms, such as a reduction in tau protein hyperphosphorylation, through the inhibition of glycogen synthase kinase 3β (GSK3β) activity
[48].
2.6.4. Antimicrobial Activity
On the other hand, quercetin has been reported to exhibit antimicrobial activity against various microorganisms. Quercetin’s proposed mechanisms in microbial infections include inhibition of bacterial growth, biofilm formation, virulence factors, and modulation of the host immune response. Quercetin has been shown to exert antibacterial effects against Gram-negative and Gram-positive bacteria, including antibiotic-resistant strains such as methicillin-resistant staphylococcus aureus (MRSA)
[51]. Recent studies have shown that quercetin can effectively alter the integrity of the bacterial cell membrane, thereby inhibiting bacterial growth. By transmission electron microscopy (TEM) analysis, Wang et al. observed that quercetin effectively altered the structural integrity of the cell wall and membrane of
E. coli and
S. aureus. In treated
E. coli, the cell wall showed numerous structural abnormalities such as prominent lysis, cell distortion, leakage of cytoplasmic fluid, and cytoplasmic membrane separated from the cell wall. Similarly, in treated
S. aureus, significant alteration of the cell wall, thinning of cell membranes, chromatin lysis, detachment of extracellular polysaccharides, and nuclear fragmentation were observed
[51][52][51,52].
2.6.5. Antiviral Activity
Several studies have suggested that quercetin may have antiviral effects against a wide variety of viruses, including hepatitis C virus (HCV), influenza A virus (IAV), Chikungunya virus (CHIKV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Inhibition of viral replication, host cell entry, and host immune response are some of the mechanisms hypothesized for the antiviral effect of quercetin. The main molecular mechanisms of quercetin’s antiviral effects are inhibition of viral neuraminidase, proteases, deoxyribonucleic acid (DNA)/ribonucleic acid (RNA) polymerases, and modification of several viral proteins. It has also been documented to suppress HCV by binding to and inactivating the viral NS3 protease
[14]. Rahman, M.A. et al. performed molecular docking in which they found that the significant interconnected nodes in the protein–protein network were protein kinase B (AKT1) (serine/threonine protein kinase), proto-oncogene tyrosine protein kinase sarcoma (SRC), epidermal growth factor receptor (EGFR), matrix metalloprotein (MMP9), kinase insert domain receptor (KDR), MMP2, insulin-like growth factor 1 receptor (IGF1R), protein tyrosine kinase 2 (PTK2), breast cancer resistance protein (BCRP), ATP-binding cassette super-family G (ABCG2), and mesenchymal–epithelial transition (MET)
[53]. Quercetin inhibits viral retrotranscriptase, as is the case with Rauscher murine leukemia virus (RMLV), human immunodeficiency virus (HIV), and hepatitis B virus (HBV)
[54][55][54,55]. Some published articles suggest that quercetin’s antiviral activity is due in part to a nonspecific protein denaturation mechanism that results in viral inactivation; however, some others offer the possibility that quercetin may bind to the surface receptors that viruses use to enter cells, thus blocking their infective capacity
[56].
2.6.6. Hepatoprotective Activity
Another essential aspect of quercetin is its hepatoprotective function. This function has been demonstrated in several animal models, specifically in models of chronic damage. Chronic liver diseases, in general, progress in a similar way; they start with a process of oxidative stress in the organ caused by a foreign agent that can be toxic to a virus, which in turn leads to an inflammatory state, which activates the processes of fibrogenesis. If the damage persists, then the disease will progress to what is known as liver cirrhosis characterized mainly by thick bundles of extracellular matrix, regeneration nodules, and necrosis
[57].
2.6.7. Oxidative Stress
Generally, at this stage, large amounts of free radicals (ROS such as superoxide anion (-O
2), hydroxyl radical (-OH), peroxyl radical (R O
2−), and alkoxyl radical (RO-) as well as RNS and ROC) are generated, which attack cell membranes, activating Kupffer cells and hepatocytes. The administration of quercetin has been shown to decrease the levels of oxidative stress associated with damage by sequestering and inactivating free radicals due to its antioxidant properties as well as by increasing the synthesis and activity of endogenous antioxidant enzymes
[58].
2.6.8. Inflammation
The inflammatory response is produced by infiltrating macrophages (inflammatory infiltrate) and liver-resident macrophages (Kupffer cells). It is characterized by the release of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor A (TNF-α), and profibrogenic cytokines, which in turn activate hepatic stellate cells (HSCs), the leading producers of the dense extracellular matrix. Experimental models have shown that quercetin can decrease the gene expression of these cytokines by downregulating their main transcriptional factor, nuclear factor κB (NFκB)
[56].
2.6.9. Fibrosis
In the chronically damaged liver by any etiology, HSCs undergo a process of activation and transformation to a proliferative, fibrogenic, proinflammatory state and express α-smooth muscle actin (α-SMA) in their cytoplasm. Among the activators reported are transforming growth factor β-1 (TGF-β1), platelet-derived growth factor (PDGF), TNF-α, connective tissue growth factor (CTGF), endothelin-1, epithelial growth factor (EGF), fibroblast growth factor (FGF), and insulin-like growth factor (ILGF). In addition, it has been shown that the administration of quercetin significantly decreases the gene expression of some of these cytokines, including TGFβ1, which is reflected in a decrease in the amount of activated HSC and the amount of extracellular matrix and collagen deposits
[59].
2.6.10. Cirrhosis
Activated HSCs produce types I, III, and IV collagen, thus initiating the fibrosis process, where the normal cells of the organ are gradually replaced by extracellular matrix, leaving few functional hepatocytes as islets in the middle of a distorted tissue. It has been reported that quercetin, in addition to preventing the development of liver fibrosis, also participates in the activation of certain antifibrogenic factors (MMP2, MMP9, and TGFβ3), which leads to an improvement in the functional status of the organ and thus avoids the establishment of the pathology
[60]. On the other hand, the antiproliferative effect of this compound on activated HSCs, characterized by uncontrolled proliferation, has been described. The use of quercetin allows the arrest of these cells during the growth phase 1 (G1) of the cell cycle through the increase in tumor suppressor proteins such as protein 53 (p53) and cell cycle inhibitors protein 21 (p21) and protein 27 (p27); it also suppresses the expression of cyclin D1, D2, E, and A and promotes apoptosis through the release of cytochrome C by increasing apoptosis antigen 1(FAS) and FAS ligand
[61].