Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by José Manuel Nájera-Maldonado.
Aging causes changes in brain tissue homeostasis, thus contributing to the development of neurodegenerative disorders. Antioxidant properties of phenolic compounds are of particular interest for neurodegenerative diseases whose psychopathological mechanisms strongly rely on oxidative stress at the brain level. Moreover, phenolic compounds display other advantages such as the permeability of the blood–brain barrier (BBB) and the interesting molecular mechanisms.
- neurodegenerative diseases
- alternative treatments
- phenolic compounds
- antioxidants
- molecular mechanisms
1. Introduction
Worldwide, the number of elderly people will double in the next 35 years, leading to an increase in the incidence of neurodegenerative disorders [1]. Neurodegenerative diseases are chronic disorders of the central nervous system, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and Huntington’s disease (HD) [2]. One of the main causes of neurodegeneration is aging, which can lead to changes in the brain related to its tissue homeostasis and contribute to the development of neurodegenerative diseases. Some neurodegenerative diseases share physiopathology characteristics, such as aggregates of misfolded proteins and the formation of extracellular or intracellular plaques [2,3][2][3]. It has been observed that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could exacerbate neurodegenerative diseases as it infects the brain by binding to angiotensin-converting enzyme 2 (ACE2) receptors that are widely expressed in the brain, including on dopaminergic neurons, which, along with consequent neuroinflammation, contribute in the short and long term to the onset of disorders such as PD [4]. Furthermore, comorbidities such as diabetes and hypertension can promote these processes. In cells, mitochondria play a key role in oxidative phosphorylation during the catabolism of glucose and the generation of cellular energy. Nevertheless, they lead to the formation of reactive oxygen species (ROS) due to various alterations in the antioxidant system that oxidizes molecules, including lipids, proteins, nucleic acids (DNA/RNA), and enzymes [5]. Lipids are essential in neuronal function, acting as a barrier between the intracellular and extracellular space. They are prone to oxidation by free radicals, which alter cell permeability and facilitate the entry of molecules that cannot normally enter except through specific channels (K+, Ca2+). They might damage proteins such as enzymes or receptors, altering cellular functioning. These alterations are present in AD, PD, and HD [6]. Standard treatment for neurodegenerative diseases does not work for most patients, who require personalized treatment. Research with new drugs has not obtained favorable results, which raises the need for new therapeutic schemes that could include phenolic compounds. It has been observed that, when consumed, phenolic compounds may function as natural inhibitors of enzymes involved in glucose metabolism, particularly α-glucosidase and α-amylase [7]. Additionally, several phenolic compounds can cross the blood–brain barrier (BBB) and have important biological effects in in vitro and in vivo models of neurodegenerative diseases, which are mainly associated with their antioxidant and anti-inflammatory capacity [8] and can even improve permeability when there are BBB alterations [9].
2. Physiopathology of Neurodegenerative Diseases
One of the main characteristics of neurodegenerative diseases is the imbalance and increase in reactive oxygen and nitrogen species (RNS), which can occur due to aggregates of altered proteins [10] or altered levels of neurotransmitters, such as glutamate. Glutamate is the most important excitatory neurotransmitter in mammals, as it is involved in cognitive processes, memory, synaptic plasticity, and neuronal development [11]. Mutations in genes encoding ionotropic glutamate receptor subunits, which are critical for synaptic transmission and plasticity, are associated with intellectual disability, autism spectrum disorders, and neurodegenerative diseases. In contrast, mutations in metabotropic glutamate receptors (GluRs), which play a role in modulating neural transmission, are preferentially associated with psychiatric or neurodegenerative disorders. The metabotropic receptors mGluR1 and mGluR5 Group I are coupled to the activation of Gq/11 proteins, which couple to signaling pathways that can affect protein kinase activation and stimulate Ca2+ release from neuronal stores (endoplasmic reticulum), triggering cell death processes [12]. Glutamate concentrations at the intracellular level are in the millimolar range, while at the extracellular level, they are at micromolar concentrations. Excess glutamate causes neuroinflammation and excitotoxicity in vitro and in vivo by the overactivation of ionotropic receptors and, as a result, contributes to neuronal death by apoptosis and autophagy [13]. Glutamate can reduce the cell survival rate by up to 60% compared to the control group in mouse hippocampal neuronal HT22 cells. Furthermore, glutamate treatment increases ROS levels [14]. The increase in ROS is due to increased Ca2+, as it can elevate mitochondrial overload, nitric oxide production, lipid peroxidation, and cytochrome C dissociation [15,16][15][16]. The use of phenolic compounds has been proposed to reduce oxidative stress and mitigate some of the alterations generated by the deregulation of the redox system since the compounds can eliminate superoxide, peroxide, singlet oxygen, hydroxyl radical, nitric oxide (NO), and peroxynitrite (OONO-), which are constantly produced in cells because of cellular respiration [17,18][17][18]. Damage to cell populations of astrocytes and neurons leads to the release of glutamate into the extracellular space, exacerbating neurodegenerative pathologies. Currently, there are a few drugs approved for the treatment of neurodegenerative diseases. For example, in the handling of AD, there are three classes of approved medications, cholinesterase enzyme inhibitors, and N-methyl D-aspartate (NMDA) antagonists, which only alleviate the symptoms of AD but do not cure or prevent the disease [19]. In January 2023, the FDA approved a new treatment with a human anti-beta amyloid (Aβ) monoclonal antibody, which is effective in reducing Aβ accumulation and slowing cognitive decline [20]. HD is caused by the expression of the mutant huntingtin protein, and these proteins can interact with hydrogen peroxide, glutathione, and, especially, copper, which promotes the oxidation of the N171 huntingtin fragment and leads to its oligomerization (there is an average of one copper for every three molecules of the huntingtin N171 protein) [21]. Additionally, in HD, alterations can be found in neuronal myelination, leading to attempted remyelination by oligodendrocytes. Iron is critical for oligodendrocyte differentiation and proliferation processes and is dependent on high iron stores. Studies in HD patients and healthy patients have shown an increase in iron in certain brain regions, such as the striatum [22]. In neurodegenerative diseases, inflammation is caused by protein aggregation or by the effect of autoimmunity, leading to an increase in cytokines, chemokines, and ROS [23,24][23][24]. Some of the most common alterations in the physiopathology of neurodegenerative diseases are shown in Figure 1. It has been demonstrated that natural and synthetic phenolic compounds can scavenge free radicals and chelate transition metals, halting progressive oxidative damage [25].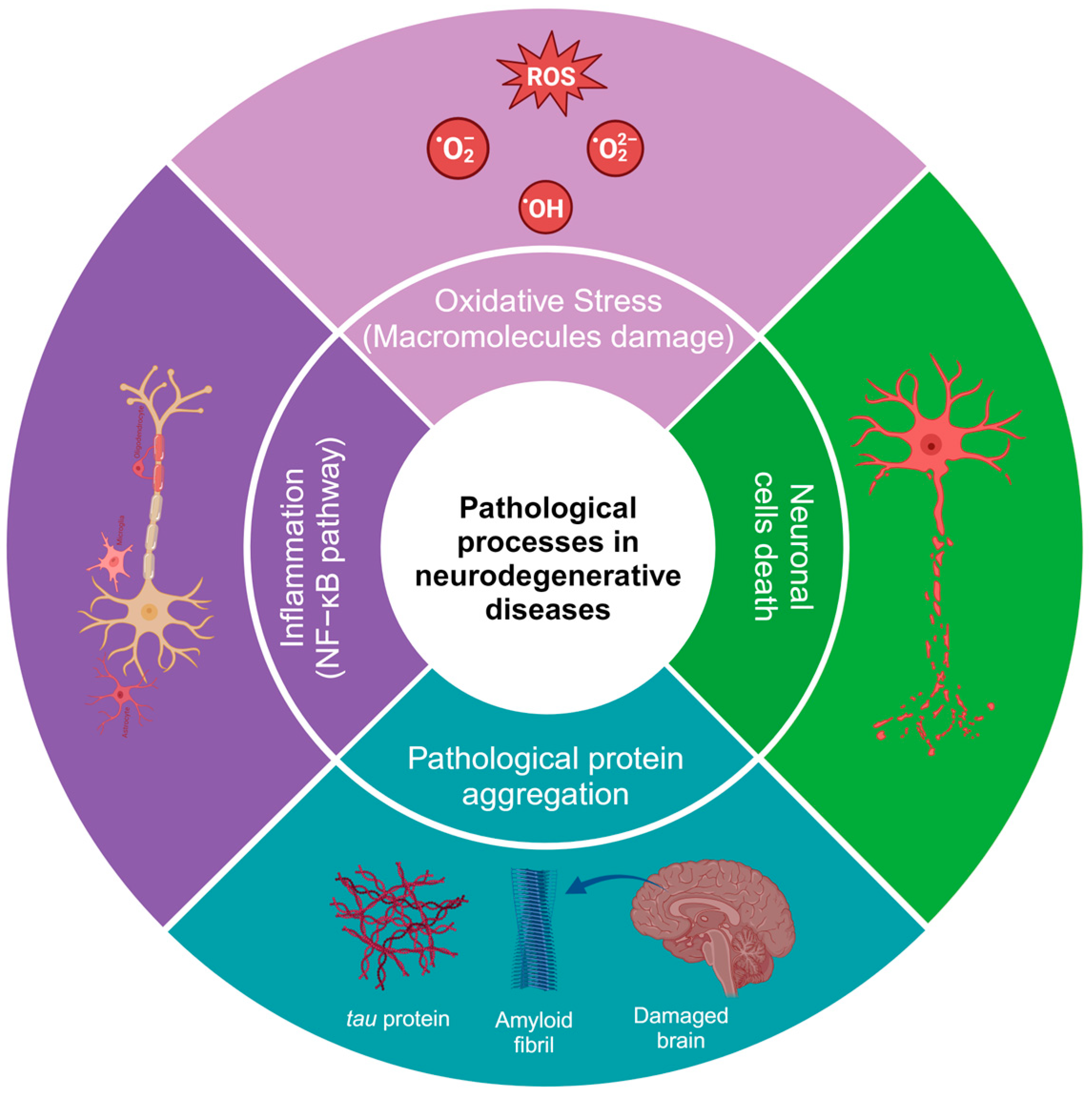
Figure 1.
Pathological processes in neurodegenerative diseases. The image was created in BioRender (
, accessed on 25 January 2024).
3. General Aspects of Phenolic Compounds
Phenolic compounds are natural metabolites that consist of at least one aromatic ring (benzene) with one or more hydroxyl substituents. They can be as simple as pyrogallol or as complex as tannic acid. The main classification criteria for phenolic compounds rely on (a) the number of rings and (b) the substitution patterns of the ring [26]. Based on the number of rings, phenolic compounds are classified as simple phenols, which can have one (single phenols) to two rings (biphenols), and polyphenols (>2 rings) [27]. Simple phenolic compounds have a C6 skeleton, which was substituted in the “-R” group. The last can be an organic, hydroxy, carboxy, or other functional group (Figure 2). Regarding the substitution patterns to “-R”, the configurations “ortho”, “meta”, and “para” in single phenols refer to 1,2, 1,3, and 1,4 substitution patterns by two hydroxyl functional groups in the benzene ring, respectively (Figure 2) [28]. However, benzenes can also be substituted with two or three functional groups. Some examples of these patterns are more extensively illustrated in Figure 2.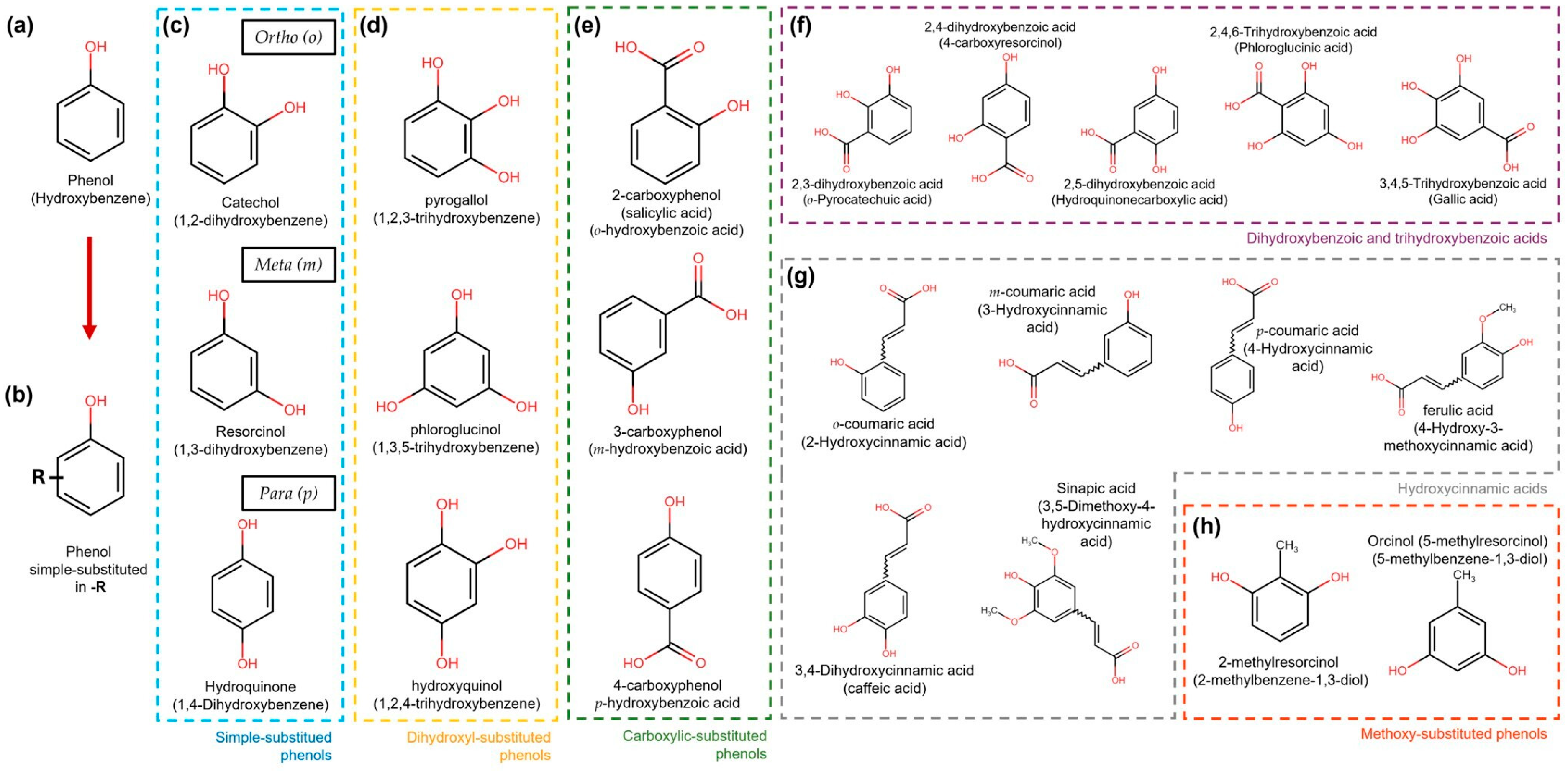
Figure 2. Simple phenol compound configurations and examples. (a) Phenol structure. (b) Simple phenolic compounds are substituted in “-R” by an organic, hydroxy, carboxy, or other functional group. (c) In simple substituted phenol compounds, also named hydroxyphenols or dihydroxybenzenes (blue dotted box), the “-R” group can be in the ortho (o), meta (m), or para (p) position of the ring. (d) Dihydroxyphenols (trihydroxybenzenes) (yellow dotted box). (e) Hydroxybenzoic acids are carboxylic-substituted phenols in o-, m- or p-positions (green dotted box). (f) Dihydroxybenzoic and trihydroxybenzoic acids are shown in the purple dotted box. (g) Phenols with the carboxylic acid functional group separated from the ring by a C=C bond are known as hydroxycinnamic acids (grey dotted box). (h) Simple phenols also can be substituted with methoxy groups (orange dotted box).
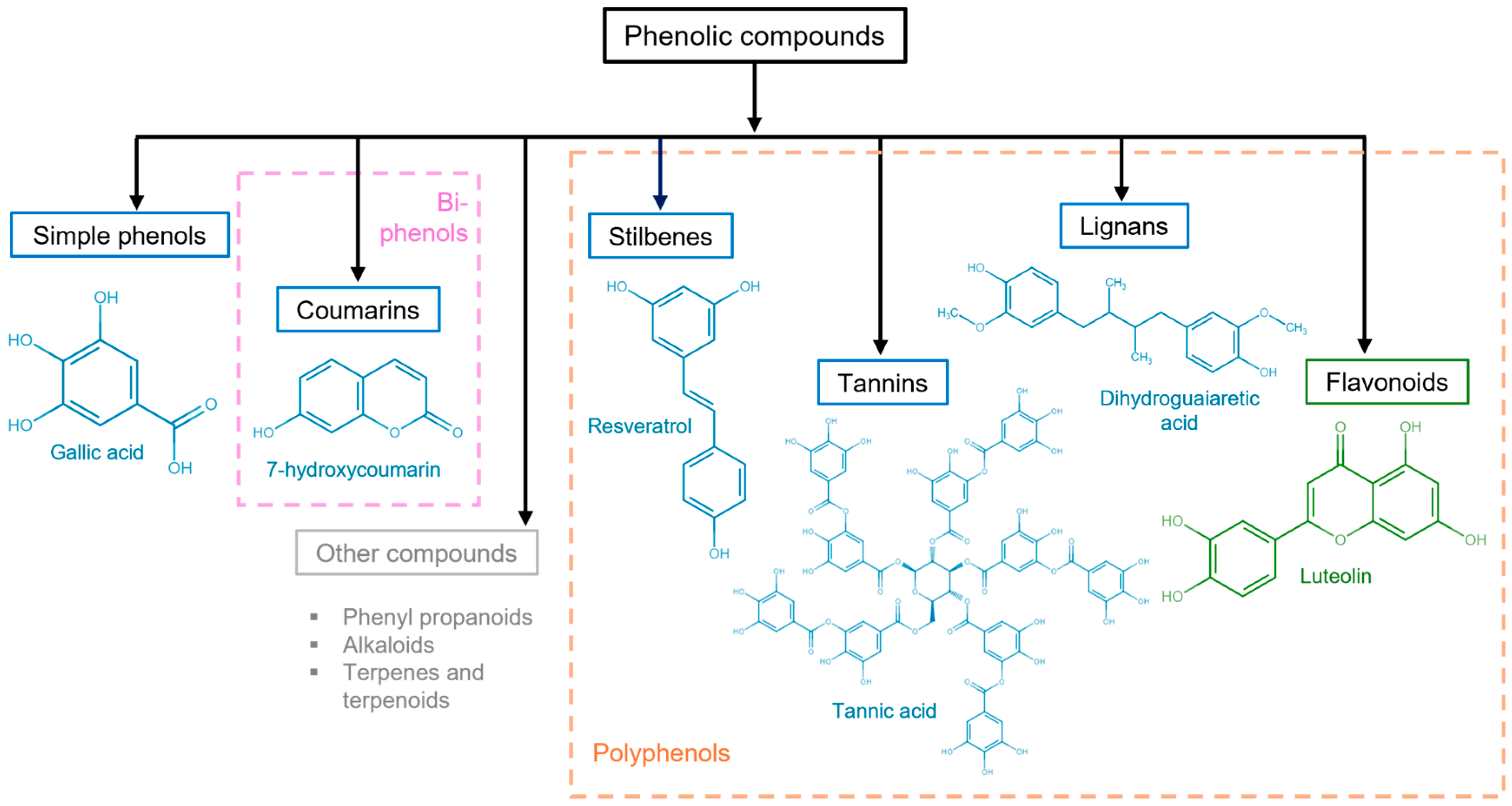
Figure 3. Polyphenolic compound classification and examples. Phenolic compounds are classified into simple phenols (see Figure 2), biphenols (pink dotted box), or polyphenols (orange dotted box). Their principal categories are phenolic acids (benzoic acids, cinnamic acids, and phenylacetic acids), coumarins, stilbenes, tannins (condensed tannins, hydrolyzable tannins, and complex tannins), and lignans, which are known as non-flavonoids (blue solid box) and flavonoids (antoxanthins and anthocyanidins) (green solid box). Other polyphenolic compounds (gray solid box).
4. Molecular Mechanisms of Phenols to Prevent Neurodegeneration
Treatment with phenolic compounds can modulate transition metal-related events in the pathophysiological processes of neurodegenerative diseases and regulate the production of ROS through different mechanisms. In HD, there are alterations in neuronal myelination, leading to an attempt at remyelination by oligodendrocytes, and iron is essential for the processes of differentiation and proliferation of oligodendrocytes, which depend on high iron stores. Natural and synthetic phenolic compounds can scavenge free radicals and chelate transition metals, halting progressive oxidative damage [21,22][21][22]. Polyphenol ligands can strongly stabilize Fe3+ compared to Fe2+. For example, in the presence of O2, the Fe2+ complexes of catecholate and gallate were rapidly oxidized to form Fe3+-polyphenol complexes [40]. Another alternative is the formation of complexes within the carbonyl group; metal ion complexes are usually formed on the C-4 keto group and the hydroxyl group on the C-5, leading to the formation of metal–flavonoid complexes. When the carbonyl group is absent, the chelation capacity of the compounds depends on the hydroxylation pattern, as the compounds tend to serve as hydrogen bridge donors [41]. The antioxidant containing the H atom reacts with the free radicals, and the free radical is stabilized to form a neutral species, while the antioxidant becomes a free radical species. The phenolic antioxidant (PA) can provide an H atom to the free radical and produce a nonradical substrate species (RH, ROH, or ROOH) and an antioxidant free radical [40]. Phenol oxidation results in the formation of quinone products of its antioxidant, and quinones can form reaction adducts of Michael addition in nucleophilic groups such as thiols and amines (Gly, Nα-acetyl-Lys, Nε-acetyl-Lys, and L-Lys) from some proteins, such as albumin [42]. Moreover, phenolic compounds can interact with proteins through noncovalent bonds comprising hydrogen bonds, hydrophobic bridges, van der Waals forces, and ionic interactions with proteins, such as with proline-rich proteins, albumin, gelatin, b-lactoglobulin, lysozyme, and serum proteins. Oxidation products of phenolic compounds such as quinones can form covalent bonds by binding to amino groups or amino acid side chains [43]. These interactions could explain why they can modulate signaling pathways involved in neurodegenerative processes and have a positive or negative effect, depending on the proteins with which they interact.Estas interacciones podrían explicar por qué pueden modular vías de señalización implicadas en procesos neurodegenerativos y tener un efecto positivo o negativo, dependiendo de las proteínas con las que interactúan.
References
- Wyss-Coray, T. Ageing, Neurodegeneration and Brain Rejuvenation. Nature 2016, 539, 180–186.
- Tian, Y.; Meng, L.; Zhang, Z. What Is Strain in Neurodegenerative Diseases? Cell. Mol. Life Sci. 2020, 77, 665–676.
- Katz Sand, I. Classification, Diagnosis, and Differential Diagnosis of Multiple Sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205.
- Ferini-Strambi, L.; Salsone, M. COVID-19 and Neurological Disorders: Are Neurodegenerative or Neuroimmunological Diseases More Vulnerable? J. Neurol. 2021, 268, 409–419.
- Wang, Y.; McLean, A.S. The Role of Mitochondria in the Immune Response in Critical Illness. Crit. Care 2022, 26, 80.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583.
- Ranilla, L.G.; Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689.
- Lopes, G.; Gomes, E.; Barbosa, M.; Bernardo, J.; Valentão, P. Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions. Molecules 2022, 27, 7707.
- Lee, B.K.; Hyun, S.-W.; Jung, Y.-S. Yuzu Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants 2020, 9, 843.
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-Beta Peptide and Tau Protein Crosstalk in Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 1666–1674.
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.W.; Olmo, R.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptors and Neurodegenerative Diseases. Pharmacol. Res. 2017, 115, 179–191.
- Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci. 2019, 12, 20.
- Jakaria, M.; Park, S.-Y.; Haque, M.E.; Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307.
- Park, D.H.; Park, J.Y.; Kang, K.S.; Hwang, G.S. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2021, 26, 1387.
- Atlante, A.; Calissano, P.; Bobba, A.; Giannattasio, S.; Marra, E.; Passarella, S. Glutamate Neurotoxicity, Oxidative Stress and Mitochondria. FEBS Lett. 2001, 497, 1–5.
- Peng, T.-I.; Jou, M.-J. Oxidative Stress Caused by Mitochondrial Calcium Overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188.
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804.
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233.
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789.
- Chowdhury, S.; Chowdhury, N.S. Novel Anti-Amyloid-Beta (Aβ) Monoclonal Antibody Lecanemab for Alzheimer’s Disease: A Systematic Review. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231209839.
- Fox, J.H.; Connor, T.; Stiles, M.; Kama, J.; Lu, Z.; Dorsey, K.; Lieberman, G.; Sapp, E.; Cherny, R.A.; Banks, M.; et al. Cysteine Oxidation within N-Terminal Mutant Huntingtin Promotes Oligomerization and Delays Clearance of Soluble Protein. J. Biol. Chem. 2011, 286, 18320–18330.
- Bartzokis, G.; Lu, P.H.; Tishler, T.A.; Fong, S.M.; Oluwadara, B.; Finn, J.P.; Huang, D.; Bordelon, Y.; Mintz, J.; Perlman, S. Myelin Breakdown and Iron Changes in Huntington’s Disease: Pathogenesis and Treatment Implications. Neurochem. Res. 2007, 32, 1655–1664.
- Yamout, B.I.; Alroughani, R. Multiple Sclerosis. Semin. Neurol. 2018, 38, 212–225.
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219.
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389.
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400.
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50.
- Mamari, H.H. Al Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In Phenolic Compounds; Badria, F.A., Ed.; IntechOpen: Rijeka, Croatia, 2021.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278.
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds; Soto-Hernandez, M., Palma-Tenango, M., del Rosario Garcia-Mateos, M., Eds.; IntechOpen: Rijeka, Croatia, 2017.
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0.
- Sharma, C.; Suhalka, P.; Bhatnagar, M. Curcumin and Resveratrol Rescue Cortical-Hippocampal System from Chronic Fluoride-Induced Neurodegeneration and Enhance Memory Retrieval. Int. J. Neurosci. 2018, 128, 1007–1021.
- Rahimi, M.; Kordrostami, M.; Mohamadhasani, F.; Chaeikar, S.S. Antioxidant Gene Expression Analysis and Evaluation of Total Phenol Content and Oxygen-Scavenging System in Tea Accessions under Normal and Drought Stress Conditions. BMC Plant Biol. 2021, 21, 494.
- Bopardikar, M.; Koti Ainavarapu, S.R.; Hosur, R.V. Pyrogallol, Corilagin and Chebulagic Acid Target the “Fuzzy Coat” of Alpha-Synuclein to Inhibit the Fibrillization of the Protein. RSC Adv. 2022, 12, 35770–35777.
- Garcia-Moreno, J.C.; de la Riva, M.P.; Martínez-Lara, E.; Siles, E.; Cañuelo, A. Tyrosol, a Simple Phenol from EVOO, Targets Multiple Pathogenic Mechanisms of Neurodegeneration in a C. Elegans Model of Parkinson’s Disease. Neurobiol. Aging 2019, 82, 60–68.
- Xie, Y.; Lu, J.; Yang, T.; Chen, C.; Bao, Y.; Jiang, L.; Wei, H.; Wu, X.; Zhao, L.; He, S.; et al. Phloroglucinol, a Clinical-Used Antispasmodic, Inhibits Amyloid Aggregation and Degrades the Pre-Formed Amyloid Proteins. Int. J. Biol. Macromol. 2022, 213, 675–689.
- Mashhadi Akbar Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19.
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Chapter 3—Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108.
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Rabadi, G.J.; Tranchant, C.C.; Almajwal, A.; Kubow, S.; Alli, I. Occurrence, Types, Properties, and Interactions of Phenolic Compounds with Other Food Constituents in Oil-Bearing Plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 3209–3218.
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394.
- Zamora, R.; Hidalgo, F.J. The Triple Defensive Barrier of Phenolic Compounds against the Lipid Oxidation-Induced Damage in Food Products. Trends Food Sci. Technol. 2016, 54, 165–174.
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-Induced Protein Modifications: Kinetic Preference for Reaction of 1,2-Benzoquinones with Thiol Groups in Proteins. Free Radic. Biol. Med. 2016, 97, 148–157.
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic-Protein Interactions: Effects on Food Properties and Health Benefits. J. Med. Food 2018, 21, 188–198.
More
