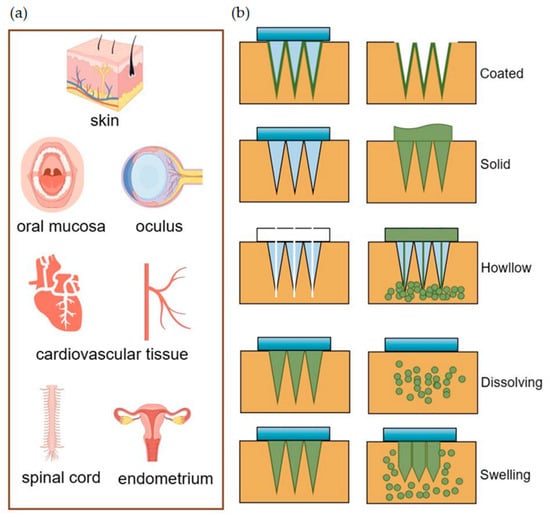
Microneedles (MNs) are microscopic needle-like structures, typically fashioned in a pyramid shape, with heights ranging from 150 to 1500 μm, widths between 50 and 250 μm, and tip thickness between 1 and 25 μm
[1][2][1,2]. They are specifically engineered to penetrate the skin’s stratum corneum, creating micron-sized mechanical channels on the surface, thereby contributing to local effective drug concentration and facilitating various medical applications
[3]. In routine transdermal drug delivery, MNs are distinguished for painless puncture
[4], scarless healing
[5], and a few gastrointestinal degradations and hepatic first-pass metabolisms
[6]. In addition, recent advancements have seen the development of MNs for targeted drug delivery to various non-cutaneous organs and tissues, such as facilitating drug entry into the oral mucosa
[7], eyeballs
[8][9][8,9], cardiovascular tissues
[10][11][10,11], spinal cord
[12][13][12,13], scalp hair follicles
[14], endometrium
[15], etc. Using MNs to deliver drugs to different anatomical sites needs to include sensitive biosensor design and miniature flexible and biocompatible electronics in the assessment
[12]. MNs can also be integrated with multiple sensors for detecting skin interstitial fluid (ISF), consistently monitoring ion concentrations, glucose, uric acid, insulin, and serotonin, providing a novel approach for disease diagnosis and prognosis monitoring
[16][17][18][16,17,18]. Considering factors such as material composition, shape, structure, mechanical strength, and biodegradability, MNs can be custom-designed for various therapeutic applications
[19]. For instance, MNs’ dressings equipped with multiple drug loads and enhanced mechanical strength not only minimize mechanical damage to non-healing wounds but also significantly expedite the wound-healing process
[20]. The programmed core–shell structure of MNs can be used to regulate the inflammatory microenvironment according to the healing stages of chronic wounds by releasing drugs in a sequential response
[21]. Extracellular vesicles (EVs), not only as messengers of cell-to-cell interstitial signaling exchange but also as carriers of multifarious bioactive molecules, nutrients, and garbage, exert various cellular life activities depending on different cellular origins
[22][23][22,23]. A specialized type of extracellular vesicles, called matrix vesicles, can induce mineral formation in bone tissues due to their abundant calcium and phosphorus content
[24]. The properties and cargo-carrying of EVs are decided by primitive cells
[22]. For instance, depending on various cell types, EVs carry cell-type-specific proteins displaying specific fates and functions
[25]. However, challenges in their application arise, as EVs repeatedly administered through intravenous injection lead to accelerated clearance in the circulatory system
[26]. Additionally, EVs applied locally often face degradation and a consequent loss of their therapeutic activity
[27]. To mitigate the accumulation of EVs in non-target organs and prevent their premature clearance, EVs are engineered to be modified by targeting their membrane components or contents
[28][29][28,29]. It is also necessary to develop continuous delivery of EVs
[30]. Thus, the availability of MNs for delivery has also been proposed as a relatively alternative method
[31]. Researchers have recently explored a range of applications utilizing MNs for the delivery of EVs to intradermal or other non-cutaneous tissues, as well as employing MNs for the detection of EVs in specific bodily or interstitial fluids
[32][33][34][35][36][32,33,34,35,36]. These advancements underscore the considerable potential of MN-mediated EVs in therapeutic and diagnostic applications for prognostic purposes in clinical settings
[37].
2. Types and Materials of Microneedles
The skin, serving as a vital protective barrier for the human body, often impedes the penetration of effective drug concentrations into the deeper tissues of wounds, particularly in cases of chronic bacterial or fungal infections caused by external injuries or immunosuppression
[38][39][40][38,39,40]. MNs, as an innovative tool in the realm of drug delivery, are designed to inject drugs locally into the epidermis, superficial dermis, or deep dermis by penetrating the skin’s stratum corneum
[41]. Research indicates that the insertion of MNs, particularly those under 1000 μm in size, avoids contact with nerve tissue and dermal blood vessels
[42]. This feature allows for the achievement of painless injections, making MNs a highly effective and patient-friendly option for localized drugs
[43]. In addition, MNs can be administered with less discomfort than conventional injections, increasing patient compliance, fastening puncture area healing, and avoiding the first-pass metabolism of orally administered drugs
[44][45][46][44,45,46]. Some MNs are prepared using specially designed materials, such as PN-Si, chitosan metal nanocomposites, etc., which are naturally antimicrobial, and thus, MNs themselves can be used as therapeutic agents to promote tissue repair
[47][48][49][47,48,49]. In addition, MN-based biosensors effectively capture dermal interstitial fluid (ISF)
[50] and have been shown in preclinical experiments to be effective in monitoring blood glucose
[51], electrolyte levels
[52], Ph level
[53], and biomarkers such as epidermal growth factor receptor 2
[54], carcinoembryonic antigen
[55], cystatin C, etc.
[56]. According to different drug release mechanisms, MNs are mainly categorized as coated, solid, hollow, dissolved, and soluble
[57]. As shown in
Figure 1, they have a wide range of tissue administrations.
Figure 1.
(
a
) MNs deliver drugs for transdermal and non-transdermal applications. (
b
) Drug diffusion diagram of different types of MNs.
The resealing time of human skin following MN treatment varies from 3 to 40 h, which can effectively enhance skin permeability for a viable duration, thereby facilitating improved drug absorption
[58]. Coated MNs are applied by puncturing the skin and delivering the drug coated on their surface to the puncture site. However, an MN patch with a size of about 10–20 cm
2 can only be coated with up to 1 mg of the drug, which constrains its ability to deliver effective drug concentrations for certain applications
[59][60][59,60]. Enhancing the coating process to achieve a uniform coating can increase the maximum drug-loading dose of MNs without compromising the drug’s activity
[61]. This improvement offers promising prospects for the large-scale production and utilization of MN patches
[62][63][62,63]. The application of solid MNs involves a two-step process: initially, they pierce the skin to create a channel, followed by the application of a topical preparation
[64]. However, solid MNs, along with hollow MNs, are susceptible to needle tip breakage, with the potential for the broken needle fragments to remain embedded in the skin tissue, posing biocompatibility issues
[59][65][59,65]. Research indicates that the use of solid MN rollers to puncture the skin, followed by the application of EVs derived from human umbilical cord mesenchymal stem cells (HUC-MSCs), can effectively alleviate the symptoms of melasma patients, offering an enhanced experience for the patient
[66]. Hollow MNs are similar in principle to the routine use of syringes and have clinical advantages in simple steps for doctors to follow
[67]. As the name suggests, hollow MNs have holes in the tip and utilize a mechanism of “poke-and-flow” to deliver the drug to the skin tissue, which allows for high-dose administration compared to other types of MNs. However, a notable disadvantage is that these needles possess relatively weak mechanical strength, making them prone to breakage, and the rate of drug delivery can lead to obstructions within the lumen
[3][68][3,68]. Dissolving MNs dissolve after insertion into the skin, and the drug is released with hydrolyzed or enzymatically dissolved polymers, which sustainably maintain the drug concentration and show promise toward wounds requiring prolonged healing
[69]. Additionally, Han and colleagues have employed digital light processing and 3D printing techniques to fabricate polymeric MNs with barbed tips aimed at enhancing tissue adhesion and maintaining the duration of drug release
[70]. The swelling MNs consist of crosslinked hydrogels that swell upon insertion into the skin by absorbing water, and the hydrogels have the ability to adapt to and mimic mechanical changes over time with a high drug loading capacity and adjustable drug release rate, overcoming the limitations of conventional MNs
[71][72][71,72]. You and their team have demonstrated that the delivery of human dermal fibroblast-derived EVs using hyaluronic acid MN patches effectively reduces skin wrinkles
[34]. Additionally, the loading of these patches with EVs-encapsulated collagen mRNAs leads to sustained collagen implantation in the dermis, further enhancing the improvement of skin wrinkles
[34].
Materials used to prepare MNs include silicon
[73], metal
[6], ceramics
[74], silica glass
[2], silk proteins
[75], polymers
[76], etc., which have been summarized in detail in many reviews
[3][27][77][3,27,77]. Polymer MNs have received more attention in recent years, and this section will focus on new advances in the preparation of MNs from this material.
A diverse range of polymers is utilized in the fabrication of MNs, commonly encompassing soluble polymers such as sodium hyaluronate, sodium carboxymethylcellulose, polyvinyl alcohol, polyvinylpyrrolidone, and degradable polymers such as polylactic acid, chitosan, poly or polylactic acid-hydroxyacetic acid copolymer, etc.
[78]. In comparison to MNs made from other materials, the mechanical properties and drug release characteristics of polymer-based MNs can be tailored by modifying factors such as the cross-linking density, concentration, molecular weight, and charge properties of the polymers
[79]. Soluble MNs are predominantly used in in vivo settings and recognized for their capability to quickly release drugs or vaccines within the body
[80]. An example of this is noted in a study by Ito et al., where it was observed that using dextrin as a matrix material for MNs facilitated the release of almost the entire quantity of formulated insulin within just one hour
[81][82][81,82]. Designing MNs with a dual-layer structure, comprising a matrix layer and a backing layer, enhances their drug delivery capabilities
[83]. The matrix layer allows for rapid administration to achieve the effective dose, while the backing layer continuously replenishes the drug
[84]. This design supports prolonged, effective drug release, contributing to a stable therapeutic environment
[85]. Patricia G-V and colleagues developed dissolvable MNs for treating neonatal sepsis, utilizing sodium hyaluronate and polyvinylpyrrolidone as the materials for the matrix and the backing layers of the MNs, respectively
[86]. In this study, gentamicin was initially administered from the needle’s tip, followed by the sustained release of the antibiotic from the backing layer through the created pores, ensuring continuous drug delivery
[86]. Zhang et al., prepared hydrogel-structured modular MNs for multiple delivery of antibiotics, IL-4, and TGF-β for the treatment of periodontitis, in which the basement membrane of the MNs rapidly dissolved to release the antibiotics, and silica particles and biodegradable nanoparticles acted as the carriers for the drugs and cytokines in the hydrogel structure of the MNs, slowly releasing the encapsulated contents
[87]. Similarly, the encapsulation of successfully isolated EVs derived from human adipose stem cells into the tips of MNs crafted from polymeric materials has been shown not only to preserve their molecular activity over an extended period but also to effectively regulate the release of these EVs
[88].
Hydrogels are three-dimensional porous polymer networks prepared by the physical or chemical cross-linking of hydrophilic molecules, and one of the most commonly used polymers is poly methyl vinyl ether-co-maleic acid cross-linked with polyethylene glycol
[89]. The hydrogels undergo swelling upon insertion into the skin without dissolving. Further, they have chemical and mechanical properties similar to those of human tissues, are biocompatible, soft, and stretchable, and can be self-healing
[90][91][92][93][94][90,91,92,93,94]. Hydrogels find extensive applications in fields such as tissue engineering, sensor fabrication, drug delivery, and biological research. It is capable of providing an intelligent drug delivery system that responds to various types of stimuli, such as temperature
[95][96][97][95,96,97], light
[98], Ph
[99][100][99,100], glucose concentration
[101], active oxygen species
[102], etc. Compared with other types of MNs, hydrogel MNs have the outstanding advantages of significantly higher drug loading and intelligent control of drug release rate
[103][104][103,104]. Guo et al., prepared glucose-responsive hydrogel-based MN dressings with strong adhesion to diabetic wounds and responsive release of insulin to different concentrations of glucose, accelerating the diabetic wound healing process, reducing inflammatory response, and realizing intelligent drug release
[105]. In addition, utilizing the water absorption and swelling characteristics, Razzaghi et al., designed 3D-printed hydrogel MNs arrays of polyethylene glycol diacrylate material integrated with multiple sensors, which allowed for the extraction of biomarkers from interstitial fluids and colorimetric diagnostic assays in a few minutes
[106]. The multifunctionality and unique properties of hydrogels make them ideal systems for biomedical applications, and the preparation of soft dressings from hydrogel MNs combined with EVs is currently a research hotspot for wound repair
[107]. While polymers and hydrogel materials demonstrate promising application prospects, current research often transcends the use of a single, simple polymer for MN fabrication
[108]. Instead, researchers are exploring related modifications or combinations of various materials to achieve enhanced application outcomes
[109]. For instance, the development of multifunctional MNs based on a magnesium metal–organic framework combined with hydrogel has shown significantly improved wound healing in diabetic mice
[110]. Soluble MNs were prepared by integrating textured polysaccharides into diphenyl carbonate cross-linked cyclodextrin metal–organic frameworks, which exhibited higher mechanical strength and better physical stability than MNs made of single hyaluronic acid
[111].
3. Loaded Cargos of Microneedles
On the one hand, MNs can effortlessly puncture tissues, change the local stress environment, induce skin collagen deposition and reorganization, and provide natural mechanical stimulation for tissue regeneration and wound repair
[112][113][112,113]. On the other hand, MNs fully demonstrate their potential in diabetes, superficial tumors, Alzheimer’s disease, infected wounds, contraception, and other therapeutically diverse applications by carrying a wide range of drugs, including small or large molecules, vaccines, nucleic acids, nanoparticles, EVs, and cells, among others
[12][59][114][115][116][12,59,114,115,116].
EVs were initially identified in mature reticulocytes and peripheral blood platelets as globular membrane vesicles, distinct from the small cellular fragments shed by dying and damaged cells
[117][118][117,118]. Almost simultaneously, matrix vesicles (MVs) were observed as electron-dense ‘leaf-like’ particles with ‘needle-like’ projections within an ossifying cartilaginous matrix
[119]. Thanks to the more widespread physiological contributions of EVs, advancements in our understanding of MVs have occurred mostly in parallel with associated developments in EV biology
[120]. A notable study shows that EVs from MSCs presented a clinical benefit to patients suffering from Menière’s disease by acting as a local adjuvant treatment, which is of great significance for EVs to become clinical therapeutic agents
[121]. Extensive research has revealed that EVs are abundantly present in various biological fluids, including plasma, intercellular fluid, cerebrospinal fluid, urine, sperm, bile, synovial fluid, saliva, and breast milk, as well as in malignant fluids in pathological conditions, effectively facilitating cellular communication by transmitting signals
[122][123][122,123]. Of note, EVs are considered to be one of the structural and functional components of the extracellular matrix
[23]. EVs are now characterized as bilipid membrane structures carrying cell-specific nucleic acids, proteins, lipids, and other bioactive molecules. These molecules bind specifically to target cells, altering the structure and function of the recipient cell
[124]. Not only EVs but also non-vesicular nanoparticles can carry nucleic acids, proteins, and other bioactive molecules. Based on their size, biogenesis, secretion mechanisms, surface markers, and physiological functions, EVs are classically categorized into microvesicles, apoptotic vesicles, and exosomes (exos)
[125]. Recent studies have discovered new EVs, such as autophagic EVs, stressed EVs, and matrix vesicles
[126]. Additional special types of EVs include membrane granules, exos-like vesicles, neutrophil-derived microvesicles, and prostasomes
[127][128][127,128]. High-resolution imaging and tracking of EVs are challenging, and specific subgroups are difficult to identify with biomarkers with 100% accuracy, leading to the potential misinterpretation of the overall effect of EVs as a heterogeneous presentation of a subgroup
[125][129][125,129]. Despite these challenges, EVs have leveraged existing isolation techniques, demonstrating significant therapeutic and companion diagnostic potential.