Ciliary beat frequency (CBF) within the ciliated epithelium of the nasal tract can be stimulated to a higher frequency and provide increased protection against transient exposure to airway irritants. Smokers as well as non-smokers exposed to secondhand tobacco smoke were found to have higher CBFs. However, with extended exposure to irritants, persistent upregulated CBF can damage and remodel the epithelial layer with fewer protective cilia. Additionally, mucociliary clearance (MCC), the innate defense mechanism of the respiratory system, traps particles and pathogens within the mucous layer of the epithelium and propels them out of the airways through ciliary activity. However, this mechanism becomes defective as disease progresses, increasing susceptibility to viral respiratory infections.
- COVID-19
- SARS-CoV-2
- smoker’s paradox
- ciliary beat frequency
- mucociliary clearance
- tobacco exposure
- ACE2
- ciliated epithelium
1. Introduction
2. Ciliated Epithelium
The human respiratory tract is lined throughout with a protective ciliated epithelium, interspersed with goblet cells that secrete a layer of mucus, as shown in Figure 1. Submucosal glands also secrete a protective airway surface liquid (ASL) across the epithelium [19][13]. Respiratory cilia are motion-producing hair-like structures that project from the apical membranes of epithelial cells in groups of 200–300 cilia per cell [20][14]. The cilium structure consists of nine microtubule doublets surrounding two central single microtubules [21][15]. Ciliary beating occurs in synchronized metachronal waves, regulated by calcium. The energy for the cilium stroke is hydrolyzed from ATP by dynein, a protein lying between the microtubule doublets which acts as a motor, putting force on the microtubules and causing the cilium to bend [22][16].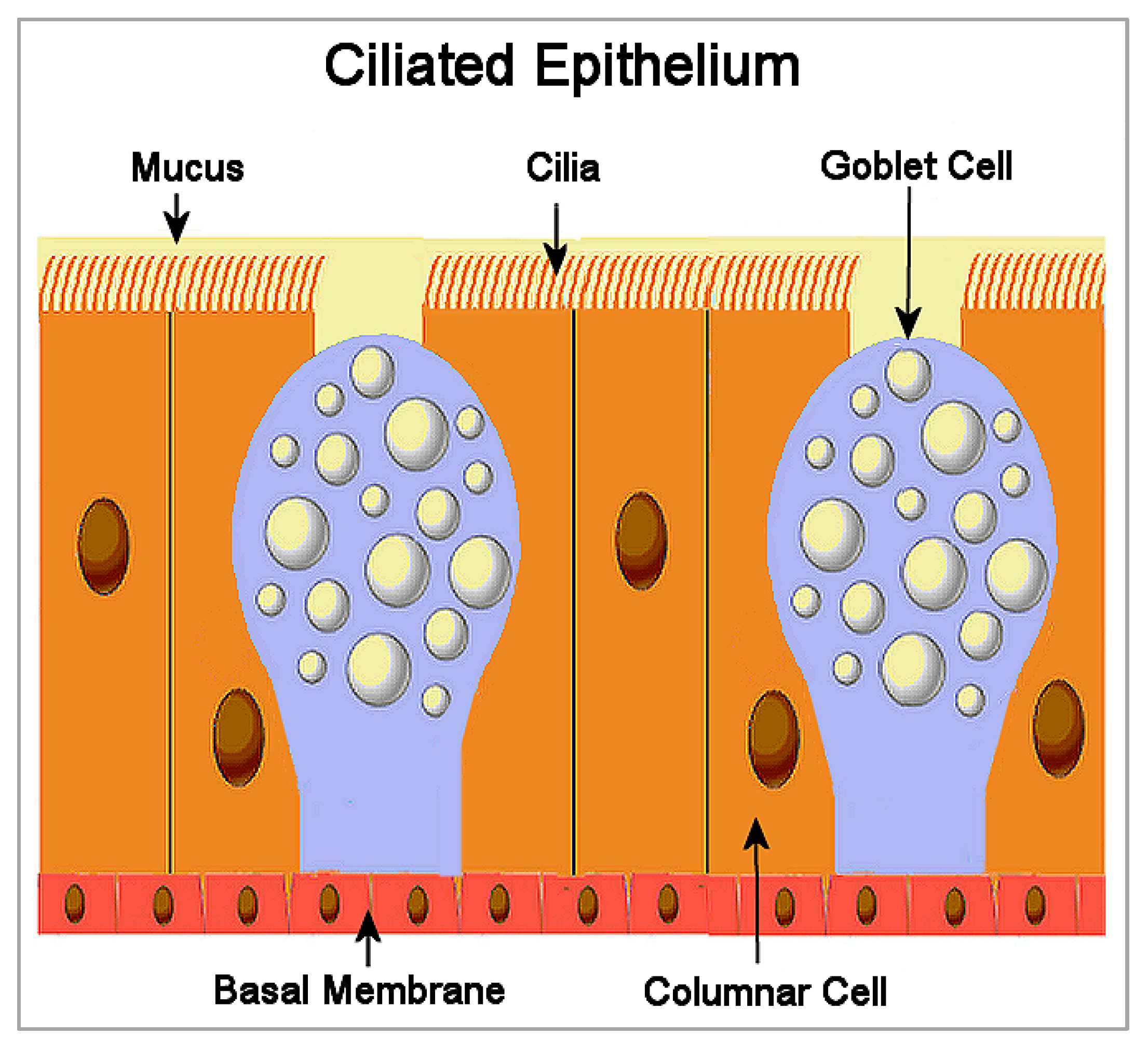
3. Mucociliary Clearance and SARS-CoV-2 Infection
Mucociliary clearance (MCC) is the innate defense mechanism of the respiratory system [28][22]. Particles and pathogens trapped within the mucous layer of the epithelium are propelled out of the airways by beating cilia. However, this mechanism becomes defective as disease progresses, with increasing susceptibility to respiratory infections involving viruses such as coronavirus, influenza, and rhinovirus [20][14]. For example, MCC was delayed in patients infected with SARS-CoV-2 compared to healthy people [29][23]. The researchers found that clearance time measured using a saccharine test was approximately 15.5 min in infected people, compared to 9.5 min in healthy people. Other studies have found that levels of ACE2 that binds with SARS-CoV-2 were more highly expressed in the nasal epithelial cells, compared to reduced levels of ACE2 expressed in the more distal bronchial epithelial cells of the lower respiratory tract [30][24]. The connection between MCC and SARS-CoV-2 infection implies that smokers with upregulated CBFs, based on the findings of Zhou et al. [23][17], are more likely to have increased MCC. This, in turn, would explain smokers’ increased odds of negative results from nasopharyngeal tests. An exception would occur in those smokers with more severe and chronic underlying respiratory diseases where the ciliated epithelium is dysfunctional and no longer protective against the accumulation of pathogens in the nasopharyngeal tract. This would explain increased risk of COVID-19 in heavy smokers. Figure 2 shows how ciliary beat frequency and mucociliary clearance is proposed to mediate the association of tobacco smoke exposure with SARS-CoV-2 nasopharyngeal infection. Depending on whether tobacco smoke exposure is transient or extended, the association between smoking and SARS-CoV-2 nasopharyngeal infection is proposed to either decrease or increase infection, consistent with the smoker’s paradox in COVID-19. Specifically, transient exposure to tobacco smoke upregulates CBF and MCC responses, and reduces SARS-CoV-2 nasopharyngeal infection. By contrast, extended exposure to tobacco smoke damages the ciliated epithelium and downregulates CBF and MCC responses, which increases SARS-CoV-2 nasopharyngeal infection.
References
- Jiang, C.; Chen, Q.; Xie, M. Smoking increases the risk of infectious diseases: A narrative review. Tob. Induc. Dis. 2020, 18, 60.
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460.
- Zhang, H.; Rostami, M.R.; Leopold, P.L.; Mezey, J.G.; O’Beirne, S.L.; Strulovici-Barel, Y.; Crystal, R.G. Expression of the SARS-CoV-2 ACE2 Receptor in the Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 2020, 202, 219–229.
- Usman, M.S.; Siddiqi, T.J.; Khan, M.S.; Patel, U.K.; Shahid, I.; Ahmed, J.; Kalra, A.; Michos, E.D. Is there a smoker’s paradox in COVID-19? BMJ Evid.-Based Med. 2021, 26, 279–284.
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20.
- Lippi, G.; Henry, B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur. J. Intern. Med. 2020, 75, 107–108.
- Yanover, C.; Mizrahi, B.; Kalkstein, N.; Marcus, K.; Akiva, P.; Barer, Y.; Shalev, V.; Chodick, G. What Factors Increase the Risk of Complications in SARS-CoV-2-Infected Patients? A Cohort Study in a Nationwide Israeli Health Organization. JMIR Public Health Surveill. 2020, 6, e20872.
- Hippisley-Cox, J.; Young, D.; Coupland, C.; Channon, K.M.; Tan, P.S.; Harrison, D.A.; Rowan, K.; Aveyard, P.; Pavord, I.D.; Watkinson, P.J. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: Cohort study including 8.3 million people. Heart 2020, 106, 1503–1511.
- Meini, S.; Fortini, A.; Andreini, R.; Sechi, L.A.; Tascini, C. The Paradox of the Low Prevalence of Current Smokers Among COVID-19 Patients Hospitalized in Nonintensive Care Wards: Results From an Italian Multicenter Case–Control Study. Nicotine Tob. Res. 2020, 23, 1436–1440.
- Rossato, M.; Russo, L.; Mazzocut, S.; Di Vincenzo, A.; Fioretto, P.; Vettor, R. Current smoking is not associated with COVID-19. Eur. Respir. J. 2020, 55, 2001290.
- Van Westen-Lagerweij, N.A.; Meijer, E.; Meeuwsen, E.G.; Chavannes, N.H.; Willemsen, M.C.; Croes, E.A. Are smokers protected against SARS-CoV-2 infection (COVID-19)? The origins of the myth. NPJ Prim. Care Respir. Med. 2021, 31, 10.
- Le Bras, A. Light smoking and CVD risk. Nat. Rev. Cardiol. 2018, 15, 136.
- Widdicombe, J.H.; Wine, J.J. Airway Gland Structure and Function. Physiol. Rev. 2015, 95, 1241–1319.
- Kuek, L.E.; Lee, R.J. First contact: The role of respiratory cilia in host-pathogen interactions in the airways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L603–L619.
- Cicuta, P. The use of biophysical approaches to understand ciliary beating. Biochem. Soc. Trans. 2020, 48, 221–229.
- Ueno, H.; Yasunaga, T.; Shingyoji, C.; Hirose, K. Dynein pulls microtubules without rotating its stalk. Proc. Natl. Acad. Sci. USA 2008, 105, 19702–19707.
- Zhou, H.; Wang, X.; Brighton, L.; Hazucha, M.; Jaspers, I.; Carson, J.L. Increased nasal epithelial ciliary beat frequency associated with lifestyle tobacco smoke exposure. Inhal. Toxicol. 2009, 21, 875–881.
- Fu, Y.; Tong, J.; Meng, F.; Hoeltig, D.; Liu, G.; Yin, X.; Herrler, G. Ciliostasis of airway epithelial cells facilitates influenza A virus infection. Vet. Res. 2018, 49, 65.
- Brown, R.B. Sodium Toxicity in the Nutritional Epidemiology and Nutritional Immunology of COVID-19. Medicina 2021, 57, 739.
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am. J. Respir. Cell. Mol. Biol. 2018, 58, 157–169.
- Hadar, T.; Yaniv, E.; Shvili, Y.; Koren, R.; Shvero, J. Histopathological changes of the nasal mucosa induced by smoking. Inhal. Toxicol. 2009, 21, 1119–1122.
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241.
- Koparal, M.; Kurt, E.; Altuntas, E.E.; Dogan, F. Assessment of mucociliary clearance as an indicator of nasal function in patients with COVID-19: A cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021, 278, 1863–1868.
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 2020, 182, 429–446.e14.
- Oran, D.P.; Topol, E.J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med. 2020, 173, 362–367.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615.
- CDC. Health Effects of Secondhand Smoke. Centers for Disease Control and Prevention—Smoking & Tobacco Use. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/secondhand_smoke/health_effects/index.htm (accessed on 29 January 2022).
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558.
