Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ronald B. Brown and Version 2 by Peter Tang.
Sudden Infant Death Syndrome (SIDS) occurs unexpectedly in an otherwise healthy infant with no identifiable cause of death following a thorough investigation. A general hypervolemic state has been identified in SIDS, and fluid in the lungs suggests the involvement of pulmonary edema and hypoxia as the cause of death.
- sudden infant death syndrome
- SIDS
- pulmonary edema
- hypoxia
- sodium chloride
- hypervolemia
- fever
- alveolar epithelium
- microvascular endothelium
1. Introduction
Sudden infant death syndrome (SIDS) is currently defined by the World Health Organization (WHO) as “the abrupt and unexplained death of an apparently healthy infant under one year of age, remaining unexplained after a thorough case investigation, including performance of a complete autopsy, examination of the death scene, and review of the clinical history” [1]. Accidental suffocation/asphyxia and deaths due to uncertain circumstances are listed separately from SIDS, under sudden unexpected infant death (SUID) [2]. This diagnostic shift reassigns asphyxia or undetermined causes to the sleep environment, which “serves to underestimate the actual mortality of what was once considered SIDS” [3].
Autopsy findings of SIDS cases include increased brain weight, possibly caused by cerebral edema “secondary to hypoxia/anoxia or toxic/metabolic factors” [4]. However, further postmortem examinations suggest that environmental toxins, including lead, mercury, and arsenic, are not a cause of SIDS [5]. On the other hand, sodium toxicity, the toxic effect from acute salt poisoning [6] or from prolonged intake of excessive dietary sodium [7], is an unexamined toxic factor potentially involved in SIDS.
Excessive dietary salt intake (sodium chloride) is strongly associated with hypervolemia, fluid overload [8], and blood–brain barrier dysregulation [9]. Of relevance, “abnormalities of regulation of the blood brain barrier with disturbances in water homeostasis” could contribute to increased brain weight in SIDS, although possible causes remain controversial [10]. Other organs with increased weight in SIDS include the thymus, liver, and lungs [4].
2. Alveolar Epithelial Permeability
Noncardiogenic pulmonary edema is unrelated to the increased vascular hydrostatic pressure in cardiogenic pulmonary edema usually seen in heart failure. In noncardiogenic pulmonary edema, disruption of the microvascular endothelial barrier increases permeability to fluid, which then fills the interstitium. Disruption of the alveolar epithelial barrier additionally increases permeability to interstitial fluid that floods the alveolus [11][20]; see Figure 1. This influx of edematous fluid may contain red blood cells [12][21].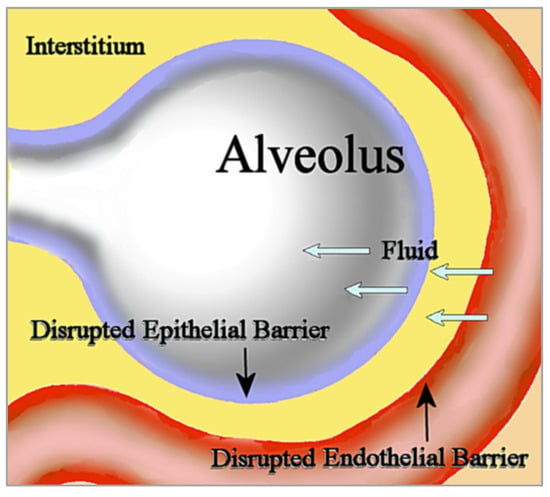
Figure 1. In noncardiogenic pulmonary edema, fluid flows into the interstitium through the disrupted microvascular endothelial barrier, then subsequently flows into the alveolus through the disrupted epithelial barrier.
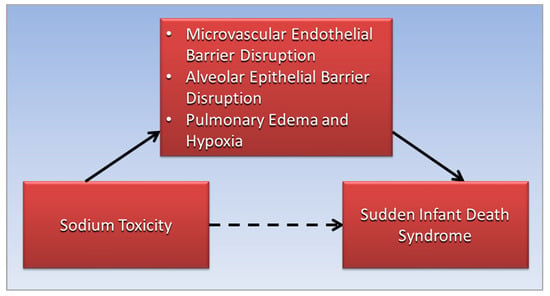
Figure 2. The association of sodium toxicity with sudden infant death syndrome (dotted arrow) is mediated (straight arrows) by disruptions of the microvascular endothelial and alveolar epithelial barriers in the lungs, leading to pulmonary edema and hypoxia (straight arrows).
3. Sodium Channelopathies and SIDS
Impaired contraction of skeletal respiratory muscles, thought to contribute to respiratory failure in SIDS, is caused by variants in the voltage-gated sodium channel NaV1.4, which is encoded by the SCN4A gene. A case-control study found that variants of SCN4A caused dysfunctional sodium channels in SIDS cases but not in controls [18][36]. Disturbances in sodium and water homeostasis commonly occur in severely ill neurology patients, and “have a profound effect on the brain” [19][38]. This effect suggests a potential link between dysfunctional sodium channelopathies in the brain and brainstem alterations thought to affect respiration in SIDS, such as reduced levels of serotonin 5-HT neurotransmitter [20][39], although more research is needed in this area. Similar to sodium channelopathies in SIDS that affect respiratory muscles and arrhythmias, future studies should investigate whether brainstem alternations in SIDS are a side effect of sodium toxicity rather than the key pathophysiological mechanism leading to respiratory failure.4. Hyperthermia and Fever
Hyperthermia and fever are pathological factors associated with SIDS; the bodies of deceased children with SIDS are often found hot and sweaty, suggesting that environmental conditions that cause hyperthermia, such as overclothing and heavy bedding, may be causative factors in SIDS [21][40]. However, heat stress is not a direct or significant cause of SIDS [22][41]. Importantly, whereas hyperthermia related to heat stress is an unregulated rise of core body temperature due to environmental conditions, fever is a regulated increase in internal temperature due to resetting by the hypothalamus, and is often seen in infections [21][40]. Additionally, “many SIDS infants have a history of viral illness preceding death” [23][42], and the number of cases of SIDS reported to have had weekly or more frequent “drenching’” sweats was much higher than control cases [24][43], likely related to fever in respiratory infections [23][42].5. Intrathoracic Petechial Hemorrhages
Among the distinctive features of SIDS, small blood vessel haemorrhages appear on the surface of the heart, lungs, and thymus, called intrathoracic petechiae; “the almost universal finding of intrathoracic petechiae in SIDS stands out as a poorly investigated phenomenon” [25][49]. Early investigators suggested petechiae in SIDS were caused by respiratory obstruction, although similar petechiae in infants were not found in cases of suffocation or asphyxia [26][50]. While subpleural and subpericardial hemorrhages are a sign of terminal respiratory obstruction, petechiae in SIDS may be related to other factors. Furthermore, salt induces endothelial cell secretion of the Willebrand factor, which causes hypercoagulability in blood clotting [27][51], and damage to the endothelial glycocalyx from excessive sodium impairs inhibition of platelet aggregation and adhesion, which “is thought to contribute to thrombosis formation” [28][52]. These mechanisms imply that intrathoracic petechial hemorrhages in SIDS may be associated with blood clotting in small vessels of the heart, lungs, and thymus from exposure to high salt concentrations. More research is needed in this area.6. Sodium and Near-Miss SIDS
In near-miss SIDS, subsequently categorized as ALTE (apparent life-threatening event) and more recently as BRUE (brief resolved unexplained event) [29][53], infants rescued from hypoxia where found to have “metabolic acidosis, cardiovascular instability, acute renal failure, ischaemic colitis, or acute neurological dysfunction” [30][54]. Each of these conditions in near-miss SIDS cases is potentially linked with dysregulated sodium. For example, metabolic acidosis found in rat models of salt-sensitive hypertension was associated with a high-salt diet [31][55]. Colitis in experiments with mice was exacerbated by a high-salt diet, which was suggested as being caused by inflammatory changes in the gut microbiota [32][56]. Furthermore, acute kidney injury is exacerbated by excessive salt intake [33][57], unstable cardiac status is associated with extreme hypernatremia [34][58], and neurological injury is caused by salt toxicity from rapid ingestion of massive amounts of sodium chloride [35][59]. These findings provide further support for the involvement of sodium toxicity in SIDS.7. Diet, Sodium, and SIDS
Breastfed infants have lower risk of SIDS compared to non-breastfed infants [36][60]. Of significance, human breast milk is much lower in sodium compared to cow milk [37][61], at 15 mg and 43 mg/100 g, respectively [38][62]. “Infants’ systems cannot handle the high levels of protein, sodium, and potassium of unmodified cow milk”, while infant formula based on modified cow milk “attempts to mimic the nutritional composition of breast milk” [39][63]. However, according to the World Health Organization, “formula milk marketing, not the product itself, disrupts informed decision-making and undermines breastfeeding and child health” [40][64]. By comparison, breastfed milk precludes infants’ exposure to sodium salts used in food processing [37][41][61,65], which potentially reduces risk of sodium toxicity in breastfed infants compared to formula-fed infants. As more foods and beverages are gradually introduced into the young child’s diet, intake levels of sodium, calories, and sugar “escalate upward from recommendations in toddlers, becoming even more pronounced among young children” [42][66]. Note that sodium chloride contains 40% sodium by weight. The World Health Organization recently established new benchmarks to reduce global mean sodium intake by 30%, “with the aim of achieving a target of less than 5 g of salt (i.e., <2 g of sodium) per day by 2025” [43][67]. Importantly:“Infants are less efficient than adults at excreting excess sodium, and the sodium intakes of infants should therefore be moderated. By about four months, healthy infants can begin to excrete an excessive sodium load”Consequently, increased incidence of SIDS, which peaks between 2–4 months from birth [45][69], occurs during a developmental period when infants are most vulnerable to harm from excessive sodium intake. In addition to high sodium levels, cow milk is much higher in phosphorus than breast milk, and excessive dietary phosphate has been linked to degeneration of dopaminergic neurons [46][73]. Coincidently, a recent study found degeneration of dopaminergic neurons in the majority of 36 SIDS cases, compared to no effect in control infants [47][74], a finding that is concordant with harm in SIDS from cow milk consumption. Infants’ exposure to excessive minerals in cow milk and other solid foods with potential risk associated with sodium toxicity and SIDS can be reduced by recommendation of exclusive breastfeeding during the first six months after birth, as advised by the American Academy of Pediatrics [48][75] and the World Health Organization [49][76].8. Seasonality and Socioeconomic Status in SIDS
Other dietary factors related to SIDS are associated with seasonality and socioeconomic status. Increased seasonal incidence of SIDS is associated with winter, which is concurrent with increased respiratory infections during colder months [50][77]. However, seasonal SIDS incidence is also concurrent with cold-weather changes in diet. For example, mice increase sodium intake with exposure to cold temperatures [51][78], and higher estimated salt intake is associated with daytime cold exposure in elderly humans [52][79]. Studies should investigate infants’ increased sodium intake in winter and potential associations with increased SIDS incidence. SIDS is more prevalent in populations with lower socioeconomic status (SES), according to an analysis of education, employment, and income factors [53][80]. Coincidently, dietary sodium intake is higher in children from populations with lower SES as well [54][55][81,82], leading to the inference that sodium toxicity is a potential mediating factor that may increase risk of SIDS in populations with lower SES.
