1. Biomarkers Related to Cell Division and Proliferation
The cell cycle is regulated by the activity of various cyclins and cyclin-dependent kinases (Cdks). Cyclins form a complex with Cdks, and complex formation results in the activation of the Cdk active site. Cyclins without Cdk activation have no enzymatic activity but have binding sites for some substrates
[1][34]. Cyclins are some of the most important cell cycle regulatory proteins and are linked to a specific phase of the cycle
[2][35]. Both cyclins and their associated proteins are currently the subject of intense research, as perturbations of their expression and regulation can lead to tumorigenesis
[3][36]. The majority of findings have reported on the overexpression of cyclins D and E in the development of many types of cancer
[4][37].
In many studies, p63 and CD31 are the primarily examined markers. The p63 protein in normal cells is found in the basal layer of squamous epithelium
[5][38]. Bavle et al.
[6][39] found that p63 expression rose with increased severity of dysplasia and increased expression in suprabasal cells. The studies showed that p63 is required to maintain cell proliferation. It was observed that as the severity of dysplasia rose, the proliferation rate increased; however, cell differentiation was jeopardised
[7][40]. As the disease progressed, the number of blood vessels increased and angiogenesis occurred. This is one of the factors that plays an important role in tumour growth and metastasis, providing nutrition to the developing tumour
[8][41]. CD31 protein is a marker of angiogenesis, so it was used to detect vascular changes near the epithelium. The correlation of p63 with CD31 added value to the categorisation of leukoplakic lesions in the cases of low and moderate dysplasia
[6][39].
Patel et al.
[9][42] assessed p63 expression in different grades of dysplasia and Cyclin D1 expression. Cyclin D1 is classified as a proto-oncogene.
P63 expression showed no statistically significant differences in different grades of dysplasia, and cyclin D1 showed only statistically significant differences between severe and mild grades of dysplasia. Gupta et al.
[10][43] used VEGF and CD34 as dysplasia markers. The study evaluated the percentage of VEGF immunoreactivity, the intensity of VEGF staining, and CD34 immunostaining. The expression of VEGF and CD34 increased significantly during the transition from normal oral mucosa to severe
oral epithelial dysplasia (OED)OED.
CD44—cluster of differentiation 44—is a transmembrane glycoprotein
[11][44]. Venkat Naga et al.
[12][45] used a cluster of differentiation 44 (CD44) antibody to assess the correlation between this marker and oral dysplasia grading. The authors compared four groups: control tissue, mild epithelial dysplasia, moderate epithelial dysplasia, and severe epithelial dysplasia. A comparison of the groups showed statistically significant results. It suggested that CD44 may be a useful marker for diagnosing dysplastic lesions.
Interestingly, Aravind et al.
[13][46] evaluated the osteopontin (OPN) expression in premalignant and malignant lesions. The authors observed a progressive increase in OPN expression, which was seen with increasing grades of dysplasia. Osteopontin seemed to be a promising biomarker in predicting the malignant potential of a premalignant lesion. Osteopontin, a phosphorylated sialoprotein, is a component of the mineralised extracellular matrices of bones and teeth
[14][47] that has many functions in inflammation, immune responses, wound healing, cell adhesion, and cell migration through interactions with integrins and CD44 variants
[15][48].
P53, also known as
TP53, is a gene that encodes a protein that regulates the cell cycle and, therefore, acts as a tumour suppressor, regulating cell division by stopping cells from growing and proliferating too rapidly or in an uncontrolled manner
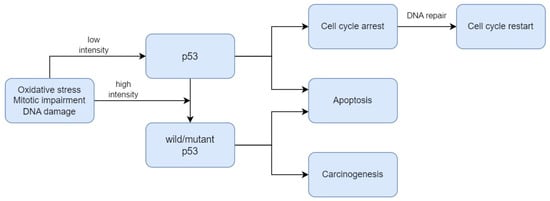
[16][49]. As presented in
Figure 1, p53 plays a critical role in the regulation of the DNA damage response. Under normal conditions, p53 is expressed at an extremely low level. The regulation of p53 activity is caused by the MDM2 protein, which contributes to the proteasomal degradation of this suppressor
[17][50]. When DNA damage or energetic stress occurs in a cell, p53 expression is induced, causing the cell cycle to stop. This is a chance for the repairment processes, or the cells will develop apoptosis. The most important purpose of this protein is to eliminate cancer-prone cells from the replication pool
[18][51]. When DNA damage, mitotic impairment, and oxidative stress are excessive, the p53 protein can be mutated to wild-type p53 protein (wtp53), which is inactivated under physiological conditions
[19][52]. Mutations in the
P53 gene and the functions of wtp53 expression have been linked to various human cancers
[16][49].
Figure 1.
The mechanism of p53 regulation in DNA damage response.
Researchers demonstrated p53’s role in differentiating grades of dysplasia. Pandya et al.
[20][53] showed that the difference in expression was statistically significant between mild and severe dysplasia. The difference in TP53 expression between mild and severe dysplasia was statistically significant, according to Patil et al.
[21][54]. The expression also increased with the increasing grades of epithelial dysplasia. Deregulation of this oncosuppressive protein may be important for the liability of the lesions to carcinogenesis. In the study by Sawada et al.
[22][55], the higher the grade of dysplasia, the more frequently a TP53 mutation was observed. Imaizumi et al.
[23][56] assessed p53 expression by immunofluorescence as a biomarker to differentiate between oral squamous epithelial lesions. The study consisted of 129 archival oral biopsy samples, including 18 benign squamous lesions, 37 low-grade dysplasias, 22 high-grade dysplasias, and 52 OSCCs. The authors found that the expression of p53 can be a valuable biomarker that helps to estimate the grade of oral epithelial dysplasia.
ΔNp63 is in the p53 family and is a p63 isoform, guiding the maturation of these stem cells through the regulation of their self-renewal and terminal differentiation. Yes-associated protein (YAP) is an oncoprotein in the cytoplasm in an inactive form
[24][57]. YAP moves to the cell nucleus and activates the transcription of genes responsible for cell division and apoptosis
[25][58]. Ono et al.
[26][59] assessed the correlation between the expression of ΔNp63 and YAP and the grade of oral dysplasia. The authors found that in oral dysplasia, the expression of YAP and ΔNp63 was higher in high-grade than in low-grade disease. YAP and ΔNp63 expression correlated with grades of oral dysplasia.
The Ki-67 protein is widely used as a marker of human cancer cell proliferation
[27][60]. Ki-67 plays a role in interphase and mitotic cells, and its distribution changes during the cell cycle. These localisations are associated with distinct functions
[28][61]. Increased tumour cell proliferation is considered a significant natural factor in cancer detection. Ki-67 plays a significant role in cancer formation due to its positive association with tumour proliferation and invasion
[29][62]. Ki-67 is the most suitable biological marker of mitotic activity due to its expression in the nucleus in a specific cell cycle period
[30][63].
Mutations of P53 and high levels of Ki-67 protein are frequently observed in various types of human cancer. Ki-67 shows a stronger association with poor tumour differentiation and negatively affects patients’ survival in advanced stages
[31][64]. Both P53 mutational status/type and high Ki-67 can also significantly impact overall survival
[32][65]. The expression of p53 and Ki-67 increases as normal oral mucosa becomes dysplastic and undergoes malignant transformation
[33][66]. Co-expression of p53 and Ki-67 is related to larger tumours and metastasis to lymph nodes; thus, this observation suggests that it can be used to identify high-risk lesions
[34][67].
In their study, Kamala et al.
[35][68] observed an increase in Ki-67 expression with the severity of dysplasia. The Ki-67 antigen can be used as a marker for histological evaluations of OED. According to Dash et al.
[36][69], as the severity of OED increases, the number of cells showing positive Ki-67 expression also increases. This is also confirmed by Mondal et al.
[37][70], who found that the differences in Ki-67 expression were statistically significant between normal mucosa and mild dysplasia, as well as between mild, moderate, and severe dysplasia. Ki-67 not only detects the hyperactive cells in OED, but its expression of Ki-67 can also be comparable to the clinical course or prognostication of a disease.
According to the study by Takkem et al.
[38][71], Ki-67 expression was restricted to the basal layers of normal oral epithelium, while Ki-67-positive cells in OED were localised in the basal, suprabasal, and squamous layers; Ki-67 expression was increased in patients at high case risk. Ki-67-positive cells in well-differentiated OSCC were mainly located at the periphery of tumour nests; in moderately differentiated OSCC, they were located both at the periphery and in part of the centre of tumour nests, while they were scattered in the most poorly differentiated lesions. The study by Kamala et al.
[35][68] aimed to determine the degree and pattern of expression of aberrant Ki67 in OSMF. The study confirmed a statistically significant correlation between the expression of Ki-67 with the clinical and histological grading of OSMF and the histological grading of OSCC.
Moreover, Swain et al.
[39][72] examined Ki-67 with MCM2 expression in OED, OSCC, and normal mucosa. The study confirmed that the expression of these proteins increased progressively. The expression profile of MCM 2 and Ki-67 was increased with the increasing grades of epithelial dysplasia. In their studies, Gadbail et al.
[40][41][73,74] used Ki-67, CD105, and α-SMA antigen to differentiate the OED grades. The expressions of Ki-67, CD105, and α-SMA markers complement the binary grading system of OED. Ki-67 showed significant increases from normal oral mucosa to low-grade and high-grade epithelial dysplasia.
Additionally, Suwasini et al.
[42][75] found a statistically significant association between p53 and Ki-67. The results highlighted the potential use of the p53 protein and the Ki-67 antigen as significant molecular markers for early PMD detection and OSCC risk. This observation was also confirmed by Leung et al.
[43][76]—Ki-67 and p53 were significantly increased with higher histological grades of OD. These observations showed the role of DNA-replicative stress in higher grades of dysplasia and transformation from OD to OSCC.
Monteiro et al.
[44][77] analysed the immunoexpression of BubR1, Mad2, Bub3, Spindly, and Ki-67 proteins in 64 oral biopsies. Spindly is a protein that targets dynein/dynactin to kinetochores in mitosis. The authors observed that the expression of Spindly was significantly correlated with a high Ki-67 score and the grade of dysplasia. This observation confirmed that the expression of Ki-67 protein is associated with an increased risk for malignant transformation.
Stathmin is a member of a family of proteins that plays important roles in regulating the microtubule cytoskeleton
[45][78]. This protein regulates microtubule dynamics by promoting the depolymerisation of microtubules and/or preventing the polymerisation of tubulin heterodimers
[46][79]. Vadla et al.
[47][80] evaluated the role of stathmin in OSCC and oral dysplasia and the correlation of stathmin expression with dysplasia grading. The study presented a statistically significant correlation between increased grades of oral dysplasia and expression levels of stathmin. This
res
earchtudy confirmed the positive role of stathmin in disease progression and suggested that stathmin could be an early diagnostic biomarker for oral dysplasia.
2. Biomarkers Related to Epithelial–Mesenchymal Transition (EMT)
Epithelial stem cells maintain tissues throughout adult life and are controlled by epithelial–mesenchymal interactions to balance cell production and loss. A defining characteristic of an epithelium is the close contact that these cells have with the underlying mesenchyme
[48][81]. Polarised epithelial cells normally interact with the basement membrane, causing several biochemical changes that enable them to adopt a mesenchymal cell phenotype, including enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and greatly increased production of ECM components. This biological process is called an epithelial–mesenchymal transition (EMT)
[49][82]. This transformation can occur in physiological processes during embryogenesis, organ development, and tissue regeneration, as well as in tumorigenesis and cancer progression, including tumour cell invasion and metastasis
[50][83].
Mesenchymal stem cells are stromal cells capable of self-renewal and multilineage differentiation. They show a greater ability to infiltrate the capillaries at the site of the primary tumour lesions
[51][52][84,85]. This mechanism is a critical mechanism for the acquisition of the malignant phenotype in neoplastic epithelial processes. This subtype accompanies the formation of distant metastases, where, in secondary foci, cells change their phenotype through a reverse mesenchymal–epithelial transition (MET)
[53][54][86,87].
The role of EMT in OSCC is to transform normal epithelial cells into malignant mesenchymal cells by losing intercellular adhesion, causing metastatic progression and infiltration
[55][88]. In the epithelial stage, tumour cells are cubic and adherent to each other. Also, in this stage, tumour cells show positive E-cadherin expression and negative vimentin expression. In the mesenchymal stage, the tumour cells show higher vimentin expression, but the expression of E-cadherin is repressed. The tumour cells are fibroblast-like and lose their cell–cell junctions
[56][89].
3. Biomarkers Related to Cell Death Regulation
Other altered proteins are the members of the Bcl-2 family. These proteins are considered as the principal players in the cascade of events that activate or inhibit apoptosis
[57][120]. In this family, there are, for example, Bcl-XL, Bcl-2, and Bax. Bcl-2 acts as a checkpoint upstream of caspases and mitochondrial dysfunction
[58][121]. Also, Bcl-2 can rescue maturation at several points of lymphocyte development. The Bcl-2 proto-oncogene was discovered at the chromosomal breakpoint of t (14;18) found in a human follicular lymphoma
[59][122]. Pathak et al.
[60][123] observed that the level of Bcl-2 increased with the grade of dysplasia. However, Bcl-2 expression was decreased in OSCC. Pallavi et al.
[61][124] assessed the expression of Bcl-2 and c-Myc in OED and OSCC. Similarly, the authors noticed that Bcl-2 increased with grades of dysplasia. Bcl-2 proteins could positively affect lesion progression from premalignancy to malignancy.
Also, the PD-1/PD-L1 pathway can be a potential marker for oral dysplasia. Programmed Cell Death Protein 1 (PD-1) inhibits immune responses and modulates T-cell activity
[62][125]. Kujan et al.
[63][126] investigated the role of the PD-1/PD-L1 pathway in the development of dysplasia and OSCC. The study found that the PD-1/PD-L1 pathway can be associated with the development of OSCC and the grade of dysplasia. Programmed cell death 4 (PDCD4) functions as a tumour suppressor and an inhibitor of protein translation
[64][127]. PDCD4 expression was observed in normal oral mucosa, OED, and OSCC. Desai and Kale
[65][128] showed that the maximum expression was observed in normal oral mucosa, which reduced significantly in OED and OSCC.
Heat shock protein 27 (HSP27) belongs to the small-molecular-weight heat shock protein family and has a molecular weight of approximately 27 KDa
[66][129]. This protein protects other proteins from damage due to environmental factors such as heat, toxins, free radicals, and ischaemia
[67][130]. Karri et al.
[68][131] found that a low expression of HSP27 could be an early molecular indicator of initial dysplastic changes in normal mucosa. Conversely, the overexpression of HSP27 could be a prognostic value of malignant transformation from oral dysplasia to oral squamous cell carcinoma. Cornulin (known as C1 Orf10, or squamous epithelial heat shock protein 53) is a member of the heat shock protein 70 (HSP70) family
[69][132]. Cornulin plays an important role in the differentiation of the epidermis. The expression of cornulin causes cell cycle arrest at G1, and its downregulation plays a role in oral carcinogenesis
[70][133]. Santosh et al.
[71][134] found that cornulin expression decreased in oral dysplasia compared with normal oral mucosa and was absent in OSCC.
4. Biomarkers Related to Cellular Metabolism
A major component of the cellular response to oxygen deprivation is the transcription factor HIF-1 (hypoxia-inducible factor-1). HIF-1 consists of an HIF-1 beta unit and one of three units of HIF-1alpha, HIF-2alpha, or HIF-3alpha
[72][135]. Patel et al.
[73][136] assessed the expression of HIF-1alpha in OED and compared the expression between grades. The authors noticed that the expression of HIF-alpha statistically significantly increased as grades of oral dysplasia were higher. Also, HIF-alpha could be a marker of risk of malignant transformation.
Inducible nitric oxide synthase (iNOS) is an enzyme in oxygen and nitrogen metabolite metabolism
[74][137]. Using immunohistochemical methods, Singh et al.
[75][138] compared iNOS expression between oral leukoplakia and OSCC. The authors found that the expression of iNOS rose with the progressing clinical stages of oral leukoplakia and OSCC. Therefore, iNOS might be a diagnostic marker in oral leukoplakia and a prognostication marker of OSCC. Another enzyme, cyclooxygenase (COX or prostaglandin–endoperoxide synthase), is required to change arachidonic acid to prostaglandins
[76][139]. Sharada et al.
[77][140] examined the expression of COX-2 and type IV collagen in OED. The study found that its expression increased significantly as the grade of dysplasia was higher. This marker could be applied to assess the malignant potential.
5. Biomarkers Related to Extracellular Signalling Pathways
Paxillin is a 68 kDa, phosphotyrosine-containing protein that may play a role in several signalling pathways
[78][141]. The study by Alam et al.
[79][142] presented a statistically significant correlation between increased grades of oral dysplasia and expression of paxillin. Paxillin may play an important role in the pathogenesis of oral dysplasia and OSCC.
EGFR is a 170 kDa transmembrane glycoprotein receptor
[80][143]. EGFR regulates cell growth, differentiation, and gene expression
[81][144]. Fakurnejad et al.
[82][145] demonstrated that an anti-EGFR agent could successfully discriminate high-grade dysplastic lesions from low-grade dysplasia. Melanoma inhibitory activity (MIA) and MIA2 are other receptors participating in tumour growth and invasion. Kawai et al.
[83][146] evaluated MIA and MIA2 as expressed in the oral mucosa within early neoplastic lesions and suggested that MIA and MIA2 are useful novel immunohistochemical markers for discriminating between normal tissue and OED.
Laminins are another family of structural proteins. Laminins participate in organising the complex interactions of the basement membranes. Laminin-1 is in the Reichert membrane (extraembryonic basement membrane)
[84][147]. A study by Vageli et al.
[85][148] assessed laminin immunostaining in biopsies as a useful biomarker of actinic cheilitis and differential diagnosis between actinic cheilitis and lip cancer. This marker can differentiate between low- and high-grade dysplasia. This
res
earchtudy can provide new insight into the mechanism of progression of actinic cheilitis into lip cancer. Also, Nguyen et al.
[86][149] evaluated the immunoexpression of LAMC2. The expression of LAMC2 was significantly associated with the grade of dysplasia. LAMC2 may be a predictive marker for the malignant progression of leukoplakia.
In the study by Debta et al.
[87][150], GLUT-1 also appeared as a marker for differentiating dysplasia severity. A statistically significant increasing level of GLUT-1 corresponded to more advanced grades of dysplasia and was consistent with the WHO system. GLUT-1 expression was significantly increased from normal to mild, moderate, and severe dysplasia. The expression of the GLUT-1 marker complemented the WHO grading system of OED. Also, Patlolla et al.
[88][151] confirmed a significant correlation between the location of GLUT-1 within the cell and the grade of dysplasia.
Moreover, Udompatanakorn and Taebunpakul
[89][152] assessed the pattern of expression of METTL3 in OED. METTL3 is an enzyme involved in the post-transcriptional methylation of internal adenosine residues
[90][153]. The authors observed that the expression of METTL3 increased in oral dysplasia and OSCC. METTL3 expression might be a marker for the progression of oral dysplasia and transformation to OSCC.
Another marker is the minichromosome maintenance protein (MCM-2), which is a key component of the pre-replication complex. This protein may be involved in the formation of replication forks and in the migration of other proteins during DNA replication
[91][154]. The study by Zakaria et al.
[92][155] aimed to assess MCM-2 activity in oral epithelial dysplastic lesions. The MCM-2 immunostaining showed a statistically significant increase from mild to severe dysplasia, and the highest value was in invasive squamous cell carcinoma. MCM-2 activity is associated with the grade of dysplasia. This observation suggests that MCM-2 may be a potential biomarker for early squamous cell carcinoma.