Prunus spinosa L. is a perennial, thorny shrub, highly decorative for landscape and forest edges, belonging to the Rosaceae family, genus Prunus, representing one of the ancestors of P. domestica. Modern phytotherapeutics emphasizes the benefits of consuming parts or products based on the Prunus spinosa L. shrub, as it is considered a plant with functional nutritional and therapeutic properties, remarkable in various pathologies with increasing incidence. Up to now, research has shown that the polyphenols found in large amounts in the fruits of P. spinosa L. are biofunctional compounds. These include anthocyanins, phenolic acids, flavonoids, and coumarin derivatives.
- Prunus spinosa L.
- phenol compounds
- oxidative stress
1. Introduction
2. The Bioecology of the P. spinosa L. Shrub
Prunus spinosa L. is a perennial, thorny shrub, highly decorative for landscape and forest edges, belonging to the Rosaceae family, genus Prunus, representing one of the ancestors of P. domestica [6,23,26][6][17][18]. It is native to Europe (Figure 1), mainly central and southern Europe, except the lower half of the Iberian Peninsula, extending northwards to the southern part of the Scandinavian Peninsula [26,27][18][19]. P. spinosa L. is also widespread in western Asia and northwest Africa and is locally present in New Zealand, Tasmania, and eastern North America (USDA NRCS, The PLANTS database, 2015) [28][20], the Pacific Northwest and New England in the U.S. (Figure 1). Some authors believe that it originates in the northernmost tip of the European continent, in Scotland [29][21], and is commonly found in Europe, around deciduous forests, and in the temperate areas of Asia, especially in central, northern, western, and southern Anatolia. Towards the east, it reaches Asia Minor, the Caucasus, and the Caspian Sea [30][22]. Isolated populations have been found in Tunisia and Algeria. It is widespread in the Southern Alps, Switzerland, at altitudes up to 1600 m [31][23]. Species of P. spinosa L. are also found on the slopes of wild, uncultivated areas in several regions of Bosnia and Herzegovina [32][24]. It is commonly found at forest edges and openings, on sunny, rocky slopes, in ravines and river valleys, and meadows and pastures from low plains to mountains [33,34][25][26]. In Romania, P. spinosa L., can be found in lowland, plain areas but is more abundant in hilly areas, extending to mountainous areas with altitudes of 900–1000 m, being present at the edge of agricultural lands, decorating the landscape or on abandoned pastures, as well as on the edge of oak and beech forests [26,35,36,37][18][27][28][29].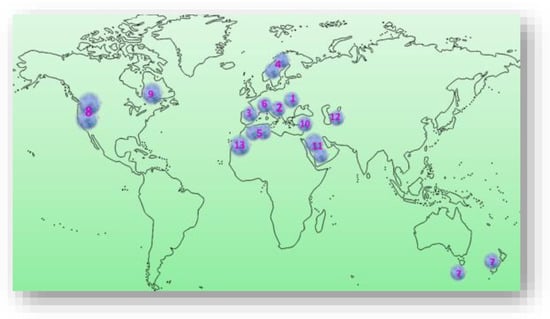
3. Nutritional composition of P. spinosa L.
The nutritional composition and estimated energy value of the blackthorn fruits are presented in Table 1.
Table 1.
Nutritional value of blackthorn fruits based on literature.
6.65 ± 2.03 | |||||
nd | 0.69 ± 0.04 |
2.72 |
1.18 ± 0.56 |
||
|
Fiber (g/100 g) |
nd |
9 |
5.79 ± 0.1 |
4.6 |
0.67 ± 0.26 |
The observed variations in the nutrient content of P. spinosa L. fruits (as shown in Table 1) can primarily be attributed to climatic conditions. Fruits grown in hot, dry climates are distinguishable by a lower level of moisture.
Differences were noted in the sugar content values (Table 1), ranging from 8.64 to 88.51 g/100 g. The variation in sugar content may be attributed to the intrinsic physicochemical properties and ripeness of blackthorn, as well as the environmental conditions [54][45].
An analysis of the amino acid composition of P. spinosa L. fruits [58][46] revealed that leucine, was found in a concentration of 122.6 mg/100 g. This amount is equivalent to 7.66% of the FAO (Food and Drug Administration) and WHO/DRIs (World Health Organization/Dietary Reference Intakes) recommended daily allowances (RDAs).Additional essential amino acids detected in P. spinosa L. were Valine (87.8 mg/100 g), Phenylalanine (84.7 mg/100 g), Lysine (50.6 mg/100 g), and Threonine (47.6 mg/100 g) [58][46].
Fatty acids found in P. spinosa L. fruits include oleic acid, linoleic acid, arachidonic acid, linolenic acid, EPA (eicosapentaenoic acid), DPA (docosapentaenoic acid) and DHA (docosahexaenoic acid) [57][47]. Fatty acid content was dominated by monounsaturated fatty acids [57][47], followed by polyunsaturated fatty acids. According to the study conducted by Babalau-Fuss (2021) [57][47] on the analysis of fatty acid content in P. spinosa L. fruits, it was found that monounsaturated fatty acids (MUFA) were the most abundant, accounting for 46.20% of the total fat content. Polyunsaturated fatty acids (PUFA) were identified in a proportion of 34.54%.
Among the mineral elements identified in P. spinosa L., potassium had the highest amount, followed by phosphorus, calcium, and sodium. The potassium levels varied between 1035.826 and 2014.23 mg/kg, the calcium levels ranged from 19.86 to 1504.41 mg/kg, and the sodium levels varied between 2.56 and 534.81 mg/kg [32,54,57][24][48][47].
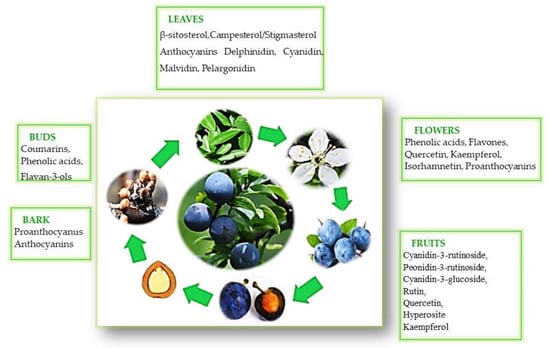
4. The Polyphenol Composition in Various Parts of the P. spinosa L. Shrub
|
Organs of P. spinosa |
Type of Sample/ Technique |
Phenols |
References |
|
Fruits |
Cold solution (1% BHT [w/v], 3% formic acid [v/v] in methanol) HPLC–DAD–MS |
Phenolic acids Cinnamic acid derivatives: · 3-p-Coumaroylquinic acid; · 4-p-Coumaroylquinic acid 1; · Caffeic acid hexoside 1; · Caffeic acid hexoside 3; · p-Coumaric acid hexoside 1; · 3-Caffeoylquinic acid; · 4-Caffeoylquinic acid; · 5-Caffeoylquinic acid 1; · 3-Feruloylquinic acid; Flavanols · Catechin; · Epicatechin; · Procyanidin dimer 1; · Procyanidin dimer 2; · Procyanidin dimer 3; · Procyanidin trimer 2; Flavonols · Quercetin triglycoside; · Quercetin acetyl hexoside; · Quercetin acetyl rutinoside; · Quercetin hexosyl pentoside 2; · Quercetin hexosyl rhamnoside; · Quercetin-3-xyloside; · Quercetin pentoside 2; · Quercetin pentoside 3; · Quercetin rhamnosyl hexoside; · Querectin-3-galactoside; · Quercetin-3-glucoside; · Quercetin-3-rhamnoside; · Quercetin-3-rutinoside; · Isorhamnetin hexoside; · Kaempferol pentoside hexoside; · Kaempferol rhamnosyl hexoside 1; · Kaempferol rhamnosyl hexoside 2; · Kaempferol pentoside; Flavones · Apigenin pentoside; Anthocyanins · Cyanidin pentoside; · Cyanidin 3-acetylglucoside; · Cyanidin-3-glucoside; · Cyanidin-3-rutinoside; · Pelargonidin-3-glucoside; · Peonidin-3-acetylglucoside; · Peonidin-3-glucoside; · Peonidin-3-rutinoside; · Petunidin-3-rhamnoside. |
[65] |
|
|
|||
|
Ethyl acetate fraction of methanol-water extract (75:25, v/v) in dried fruit UHPLC-PDA-ESI-MS |
Phenolic acids · Protocatechuic acid 4-O-hexoside; · Protocatechuic acida; · 3-O-Caffeoylquinic acid; · p-Hydroxybenzoic acida; · Caffeoylshikimic acid derivative; · Vanilloyl malate hexoside; · 3-O-p-Coumaroylquinic acid; · p-Coumaric acid O-hexoside; · 5-O-Caffeoylquinic acid; · cis-3-O-Feruloylquinic acid; · 4-O-Caffeoylquinic acid; · Caffeic acid 3/4-O-hexoside; · 3-O-Feruloylquinic acid; · Vanillina; · 4-O-Caffeoylshikimic acid; · 4-O-Feruloylquinic acid; · Caffeoylshikimic acid; · Caffeoylshikimic acid; · p-Coumaroylshikimic acid; · Aromadendrin hexoside; · p-Coumaroylshikimic acid; Flavonols · Quercetin 3-O-β-D-galactoside; · Quercetin 3-O-(6′′-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; · Quercetin 3-O-β-D-glucopyranoside; · Quercetin 3-O-α-D-xylopyranoside; · Quercetin 3-O-α-L-arabinopyranoside; · Quercetin 3-O-α-L-arabinofuranoside; · Quercetin 3-O-(4′′-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside; · Quercetin 3-O-α-L-rhamnopyranoside; · Quercetin malyl-pentoside; · Quercetin acetyl-hexoside-rhamoside. |
[24] |
|
|
Flowers |
Defatted methanol-water extract RP-HPLC-PDA |
Phenolic acids · 3-O-Caffeoylquinic acid (neochlorogenic acid); · 5-O-Caffeoylquinic acid (chlorogenic acid); · 4-O-Caffeoylquinic acid (cryptochlorogenic acid); · Caffeic acid; · p-Coumaric acid; Flavanols · (+)-Catechin; · (–)-Epicatechin; Flavonols · Kaempferol 3-O-α-L-arabinopyranoside-7-O-α-L-rhamnopyranoside; · Kaempferol 3-O-β-D-xylopyranoside-7-O-α-L-rhamnopyranoside (lepidoside); · Kaempferol 3,7-di-O-α-L-rhamnopyranoside (kaempferitrin); · Kaempferol 3-O-α-L-arabinofuranoside-7-O-α-L-rhamnopyranoside; · Kaempferol 3-O-β-D-xylopyranoside; · Kaempferol 3-O-(4’’-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside (multiflorin B); · Kaempferol 3-O-α-L-arabinofuranoside (juglanin); · Kaempferol 3-O-α-L-rhamnopyranoside (afzelin); · Kaempferol 7-O-α-L-rhamnopyranoside; · Kaempferol 3-O-(2’’-O-E-p-coumaroyl)-α-L-arabinofuranoside-7-O-α-Lrhamnopyranoside; · Kaempferol 3-O-(6’’-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside; · Kaempferol 3-O-(2’’-O-E-p-coumaroyl)-α-L-arabinofuranoside. · Kaempferol; · Quercetin 3-O-(6’’-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (rutin); · Quercetin 3-O-(2’’-O-β-D-glucopyranosyl)-α-L-arabinofuranoside; · Quercetin 3-O-β-D-glucopyranoside (isoquercitrin); · Quercetin 3-O-β-D-galactopyranoside (hyperoside); · Quercetin 3-O-α-D-xylopyranoside (reinutrin); · Quercetin 3-O-α-L-arabinopyranoside (guaiaverin); · Quercetin 3-O-(4’’-O-β-D-glucopyranosyl)-α-L-rhamnopyranoside (multinoside A); · Quercetin 3-O-α-L-arabinofuranoside (avicularin); · Quercetin 3-O-α-L-rhamnopyranoside (quercitrin); · Quercetin; |
[66] |
|
Leaves |
70% (v/v) aqueous-methanolic extract UHPLC-PDA-ESI–MS |
Phenolic acids · 3-O-caffeoylquinic acid (neochlorogenic acid); · 3-O-p-coumaroylquinic acid; · 3-O-feruloylquinic acid; · 4-O-caffeoylquinic acid (cryptochlorogenic acid); Flavanols · procyanidin type-B dimer; · procyanidin type-B dimer; · (+)-catechina; Flavonoids · kaempferol 3-O-a-L-arabinopyranoside-7-O-a-L-rhamnopyranosidea; · kaempferol 3-O-b-D-xylopyranoside-7-O-a-L-rhamnopyranoside (lepidoside); · quercetin 3-O-(200-O-b-D-glucopyranoside)-a-L-arabinofuranosidea; · kaempferol 3,7-di-O-a-L-rhamnopyranoside (kaempferitrin); · kaempferol 3-O-a-L-arabinofuranoside-7-O-a-L-rhamnopyranosidea; · quercetin 3-O-a-L-arabinofuranoside (avicularin); · kaempferol hexoside-pentoside; · kaempferol 3-O-a-L-arabinofuranoside (juglanin); · kaempferol 3-O-a-L-rhamnopyranoside (afzelin); · quercetin acetyl-hexoside-rhamnoside; · kaempferol acetyl-hexoside-rhamnoside; · kaempferol 7-O-a-L-rhamnopyranosidea; · kaempferola; · kaempferol 3-O-(2”-E-p-coumaroyl)-a-L-arabinofuranoside-7-O-a-L-rhamnopyranoside. |
[67] |
|
Branches |
Lyophilized extract HPLC/MS |
Phenolic acids · Protocatechuic acid; · Gallic acid; · Caffeic acid; Proanthocyanidins or flavan-3-ols · Ent-(epi)-catechin-(2α→O→7,4α→8)-(epi)-catechin-3′-O-gallate; · Ent-(epi)-afzelechin-(2α→O→7,4α→8)-(epi)-catechin-3′-O-gallate; · Ent-(epi)-gallocatechin (2α→O→7, 4α→8)(epi)-catechin; · Ent-(epi)-catechin (2α→O→7, 4 α→8)-catechin; · Ent-(epi)-gallocatechin (2α→O→7, 4α→8)-(epi)-catechin; · Ent-(epi)-catechin (2α→O→7, 4 α→8)-(epi)-catechin; · Ent-(epi)-afzalechin (2α→O→7, 4α→8) catechin; · Epigallocatechin; ·Ent-(epi)-afzalechin (2α→O→7, 4α→8)-(epi)-catechin; · Gallocatechin; · Epicatechin; · Catechin; · Epiafzelechin; · Afzelechin; Coumarins · 5-hydroxy-6-methoxy-7-O-β-D-glucosyl coumarin; · 5-hydroxy-6-methoxy-7-O-β-D-rhamnosyl coumarin; Flavonols · Quercetin 3-O-rutinoside; · Kaempferol 3,7-O-dirhamnoside; ·Kaempferol 3-O-arabinoside-7-O-rhamnoside; kaempferol 3-O-arabinoside; · Quercetin; · Kaempferol |
[6] |
5. The Effect of Bioactive Compounds Found in the Blackthorn Fruits in the Treatment of Various Diseases
| Bioactive Compounds | Health Effect | Main Outcomes | References |
|---|---|---|---|
| Flavonoids (Quercitin, Rutin) |
Neurogenerative effect | -acetylcholinesterase inhibition -monoamine oxidase inhibition -↓ peroxyl radical capture and oxidation -neurotrophic action -maintenance of physiological functions of vital organs |
[88,[6189]][62] |
| Flavonoids | Cardiovascular effect | -inhibition of pro-inflammatory enzymes -anti-atherosclerotic effects -anti-atherothrombotic effects -modulation of lipid metabolism, -normalization of the LDL/HDL ratio, -improving capillary permeability -improved endothelial function, vasodilatory effects -↑ release of nitric oxide and uncoupling of endothelial nitric oxide synthesis -↓ oxidative DNA damage |
[48,62,90,91,92,93,94][41][63][64][65][66][67][68] |
| Proantocianidine | -modulates lipid metabolism, -↑ anti-oxidant capacity of plasma, - improve vascular functions -↓ platelet activity |
[48,95][41][69] | |
| Cianidin-3-rutinoside | -improve lipid ↓ mechanisms, -inhibition of lipolytic digestive enzymes -inhibition of lipid absorption processes -anti-oxidant activity on ROS, -antiglycating activity -cardioprotective activity mixed competitive inhibition of pancreatic lipase and pancreatic cholesterol esterase -inhibition of cholesterol mycelial formation linked to primary and secondary bile acid -inhibition of cholesterol mycelial absorption in the proximal jejunum. |
[48,96,97][41][70][71] | |
| Cianidin-3-glucoside | -↑ tissue tolerance to ischemic injury -↓ risk of cardiovascular disease, hypertension, -capacity to scavenge ROS -↓ oxidative stress, enhancing inflammatory responses |
[96,98][70][72] | |
| Cianidin-3-glucoside Cianidin 3-rutinoside |
Diabetes and associated metabolic diseases | ↓ risk of diabetes and obesity ↓ postprandial glucose by inhibition of pancreatic α-amylase and intestinal α-glucosidase -modulates postprandial blood glucose by inhibiting carbohydrate digestive enzymes -↓ glucose transport in the small intestine. -inhibit glucose uptake in colorectal adenocarcinoma epithelial cells |
[98,99][72][73] |
| Phenolic acids | Anticancer effect | -cytotoxic activity on some cancer cell lines -induction in vitro of endogenous anti-oxidant mechanisms -modulation of Nrf2 transcription factors, a regulator of cellular resistance to oxidative damage, -↓ disruption of the pro-oxidant/anti-oxidant balance with a key role in some cancers |
[16,20,23,100][16][17][74][75] |
| Anthocyanins | -unchanged dietary absorption and incorporation into cells, -major contribution to establishing anti-oxidant activity, reducing cancer risk |
[2,15,[67,90,2101,102]][52][64][76][77][78] | |
| Phytosterols | -anticancer activity on prostate cancer | [15][76] | |
| Cianidin-3-glucozide | ↓ cancer risk due to the ability to scavenge ROS | [100][75] |
Reference |
|
[52][1] |
[37][1] |
[15][1] |
[53][1] |
[54] |
|
|
Energy (kcal/100 g) |
383.27 ± 7.09 |
57 |
154.93 |
249 |
nd |
|
Moisture (%) |
60.86 ± 1.69 |
54.85 ± 2.11 |
nd |
69.37 |
nd |
|
Carbohydrates (g/100 g dw) |
88.51 ± 2.24 |
8.64 |
31.07 ± 0.62 |
nd |
15.17 ± 25.83 |
|
Proteins (g/100 g dw) |
2.86 ± 0.03 |
0.75 |
2.07 ± 0.04 |
3.4 |
0.99 ± 0.25 |
|
Fat (g/100 g dw) |
1.98 ± 0.32 |
1 |
2.05 ± 0.12 |
2.06 |
nd |
|
Ash |
References
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; Van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046.
- Dedić, A.; Džudžević Čančar, H.; Stanojković, T.; Roje, M.; Damjanović, A.; Alispahić, A.; Jerković-Mujkić, A. HPLC Analysis of Phytosterols in Prunus spinosa L. Extracts and Their Antiproliferative Activity on Prostate Cancer Cell Lines. Kem. U Ind. 2023, 72, 323–330.
- Crnić, I.; Frančić, T.; Dragičević, P.; Balta, V.; Dragović-Uzelac, V.; Đikić, D.; Landeka Jurčević, I. Blackthorn Flower Extract Impact on Glycaemic Homeostasis in Normoglycemic and Alloxan-Induced Hyperglycaemic C57BL/6 Mice. Food Technol. Biotechnol. 2021, 59, 349–359.
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadağ, A.E.; Polat, D.Ç. Antioxidant Activity and Cytotoxic Effects of Prunus spinosa L. Fruit Extract on Various Cancer Cell Lines. MMJ 2019, 34, 297–304.
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Árvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Della Croce, C.M.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects Against In Vitro and In Vivo Oxidative Stress. Foods 2019, 9, 5.
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of in Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62.
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68.
- Marchelak, A.; Kolodziejczyk-Czepas, J.; Wasielewska, P.; Nowak, P.; Olszewska, M.A. The Effects of Prunus spinosa L. Flower Extracts, Model Polyphenols and Phenolic Metabolites on Oxidative/Nitrative Modifications of Human Plasma Components with Particular Emphasis on Fibrinogen In Vitro. Antioxidants 2021, 10, 581.
- Kotsou, K.; Stoikou, M.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Sfougaris, A.I.; Lalas, S.I. Enhancing Antioxidant Properties of Prunus spinosa Fruit Extracts via Extraction Optimization. Horticulturae 2023, 9, 942.
- Najgebauer-Lejko, D.; Liszka, K.; Tabaszewska, M.; Domagała, J. Probiotic Yoghurts with Sea Buckthorn, Elderberry, and Sloe Fruit Purees. Molecules 2021, 26, 2345.
- Ürkek, B.; Şengül, M.; Akgül, H.İ.; Kotan, T.E. Antioxidant Activity, Physiochemical and Sensory Characteristics of Ice Cream Incorporated with Sloe Berry (Prunus spinosa L.). Int. J. Food Eng. 2019, 15, 20180029.
- Egea, I.; Sánchez-Bel, P.; Romojaro, F.; Pretel, M.T. Six Edible Wild Fruits as Potential Antioxidant Additives or Nutritional Supplements. Plant Foods Hum. Nutr. 2010, 65, 121–129.
- Özkan, G. Bioaccessibility of Blackthorn (Prunus spinosa) Beverage Polyphenols: Effect of Sugar Andcitric Acid Addition. Turk. J. Agric. For. 2023, 47, 1017–1024.
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Carocho, M.; Barreira, J.C.M.; Kamal Genena, A.; José Baraldi, I.; Filomena Barreiro, M.; Barros, L.; Ferreira, I.C.F.R. Ficus carica L. and Prunus spinosa L. Extracts as New Anthocyanin-Based Food Colorants: A Thorough Study in Confectionery Products. Food Chem. 2020, 333, 127457.
- Tiboni, M.; Coppari, S.; Casettari, L.; Guescini, M.; Colomba, M.; Fraternale, D.; Gorassini, A.; Verardo, G.; Ramakrishna, S.; Guidi, L.; et al. Prunus spinosa Extract Loaded in Biomimetic Nanoparticles Evokes In Vitro Anti-Inflammatory and Wound Healing Activities. Nanomaterials 2020, 11, 36.
- Condello, M.; Pellegrini, E.; Spugnini, E.P.; Baldi, A.; Amadio, B.; Vincenzi, B.; Occhionero, G.; Delfine, S.; Mastrodonato, F.; Meschini, S. Anticancer Activity of “Trigno M”, Extract of Prunus spinosa Drupes, against in Vitro 3D and in Vivo Colon Cancer Models. Biomed. Pharmacother. 2019, 118, 109281.
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and Apoptotic Activities of Prunus spinosa Trigno Ecotype Extract on Human Cancer Cells. Molecules 2017, 22, 1578.
- Popescu, I.; Caudullo, G. Prunus cerasifera in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3.
- Prunus spinosa, L. |Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:730297-1 (accessed on 19 August 2023).
- USDA. Plants Database. Available online: https://plants.usda.gov/home (accessed on 23 November 2023).
- Fraternale, D.; Giamperi, L.; Bucchini, A.; Sestili, P.; Paolillo, M.; Ricci, D. Prunus spinosa Fresh Fruit Juice: Antioxidant Activity in Cell-Free and Cellular Systems. Nat. Prod. Commun. 2009, 4, 1934578X0900401211.
- Müller, N.; Kelcey, J.G. Plants and Habitats of European Cities; Springer: New York, NY, USA, 2011; ISBN 978-0-387-89683-0.
- Komarov, V.; Shishkin, B.; Yuzepchuk, S.V.; Fedorov, A.; Kostina, K.; Kovalev, N.V.; Krishtofovich, A.N.; Linchevskii, I.A.; Poyarkova, A.I. Flora of the USSR—Volume X: Rosaceae—Rosoideae, Prunoideae; 1970; ISBN 100706510925.
- Dedić, A.; Dţudţević-Čančar, H.; Alispahić, A.; Tahirović, I.; Muratović, E. In-Vitro Antioxidant and Antimicrobial Activity of Aerial Parts of Prunus spinosa L. Growing Wild in Bosnia and Herzegovina. Int. J. Pharm. Sci. Res. 2021, 12, 3643–3653.
- Day, J.; Robertson, P.; Symes, N. The Scrub Management Handbook: Guidance on the Management of Scrub on Nature Conservation Sites; English Nature: Wetherby, UK, 2003; ISBN 978-1-85716-745-0.
- Costa, J.; Neto, C.; Aguiar, C.; Capelo, J.; Santo, J.; Honrado, C.; Pinto-Gomes, T.; Monteiro-Henriques, T.; Sequeira, M.; Lousã; et al. Vascular Plant Communities in Portugal (Continental, the Azores and Madeira). Glob. Geobot. 2012, 2, 1–180.
- Cosmulescu, S. Gruia Marius Climatic Variability in Craiova (Romania) and Its Impacts on Fruit Orchards. South-West. J. Hortic. Biol. Environ. 2016, 7, 15–26.
- Cosmulescu, S.; Baciu, A.; Gruia, M. Influence of Climatic Factors on the Phenology Spring in Southern Oltenia (Romania). J. Hortic. For. Biotechnol. 2015, 19, 147–157.
- Andronie, L.; Holonec, L.; Pop, I.; Truta, A.M.; Odagiu, A.; Sălăgean, T.; Sobolu, R.; Coroian, A.; Balta, I.; Șuba, E.E. Antioxidant Capacity of Several Romanian Forest Fruits (Rosa canina L., Prunus spinosa L., Vaccium vitis-idaea L. and Cornus mas L.). Not. Bot. Horti Agrobot. 2019, 47, 1178–1184.
- Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 23 November 2023).
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as Probiotics and Their Implications in Food Safety. Int. J. Food Microbiol. 2011, 151, 125–140.
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune Responses to Implants—A Review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32, 6692–6709.
- Băbălău-Fuss, V.; Senila, L.; Becze, A.; Al-Zaben, O.; Dirja, M.; Tofana, M. Fatty Acids Composition from Rosa canina and Prunus spinosa Plant Fruit Oil. Stud. Univ. Babeș-Bolyai Chem. 2021, 66, 41–48.
- Cosmulescu, S.; Baciu, A.; Cichi, M.; Gruia, M. The effect of climate changes on phenological phases in plum tree (Prunus domestica L.) in south-western romania. South West. J. Hortic. Biol. Environ. 2010, 1, 9–20.
- Ruiz-Rodríguez, B.M.; Ancos, B.d.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; Sánchez-Mata, M.d.C.; Cámara, M.; Tardío, J. Wild Blackthorn (Prunus spinosa L.) and Hawthorn (Crataegus monogyna Jacq.) Fruits as Valuable Sources of Antioxidants. Fruits 2014, 69, 61–73.
- De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.d.C.; Ferino, G.; Vassallo, A.; Martelli, G.; Caddeo, C. Phenolic Fingerprint, Bioactivity and Nanoformulation of Prunus spinosa L. Fruit Extract for Skin Delivery. Pharmaceutics 2023, 15, 1063.
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenols and Maillard Reaction Products in Dried Prunus spinosa Fruits: Quality Aspects and Contribution to Anti-Inflammatory and Antioxidant Activity in Human Immune Cells Ex Vivo. Molecules 2022, 27, 3302.
- Levon, V. The Contents of Catechins and Anthocyanins in the Above-Ground Organs of Plants of Prunus spinosa L. In Agrobiodiversity for Improving of Nutrition, Health and Life Quality; Klymenko, S., Ed.; Slovak University of Agriculture: Nitra, Slovakia, 2019; pp. 265–272. ISBN 978-80-552-2108-3.
- Kuru Berk, S.; Tas, A.; Orman, E.; Gundogdu, M.; Necas, T.; Ondrasek, I.; Karatas, N.; Ercisli, S. Agro-Morphological and Biochemical Characterization of Wild Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin Genotypes Naturally Grown in Western Black Sea Region of Turkey. Agronomy 2020, 10, 1748.
- Colomba, M.; Benedetti, S.; Fraternale, D.; Guidarelli, A.; Coppari, S.; Freschi, V.; Crinelli, R.; Kass, G.E.N.; Gorassini, A.; Verardo, G.; et al. Nrf2-Mediated Pathway Activated by Prunus spinosa L. (Rosaceae) Fruit Extract: Bioinformatics Analyses and Experimental Validation. Nutrients 2023, 15, 2132.
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-Rutinoside Acts as a Natural Inhibitor of Intestinal Lipid Digestion and Absorption. BMC Complement. Altern. Med. 2019, 19, 242.
- Kültür, S. Medicinal Plants Used in Kirklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364.
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108.
- Calvo, M.I.; Cavero, R.Y. Medicinal Plants Used for Cardiovascular Diseases in Navarra and Their Validation from Official Sources. J. Ethnopharmacol. 2014, 157, 268–273.
- Ozzengin, B.; Zannou, O.; Koca, I. Quality Attributes and Antioxidant Activity of Three Wild Plums from Prunus spinosa and Prunus domestica Species. Meas. Food 2023, 10, 100079.
- Özcan, T.; Kahyaoğlu, G. Fatty Acid and Amino Acid Profiles in the Fruits of Prunus spinosa L. Subsp. Dasyphylla (Schur) Domin from Europe-in-Turkey. Adv. Mol. Biol. 2008, 1, 39–46.
- Babalau-Fuss, V.; Becze, A.; Roman, M.; Moldovan, A.; Cadar, O.; Tofana, M. Chemical and technological properties of blackthorn (Prunus spinosa) and rose hip (Rosa canina) fruits grown wild in cluj-napoca area. Agricultura 2020, 113–114, 1–6.
- Ozzengin, B.; Zannou, O.; Koca, I. Quality Attributes and Antioxidant Activity of Three Wild Plums from Prunus spinosa and Prunus domestica Species. Meas. Food 2023, 10, 100079.
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-Rutinoside Acts as a Natural Inhibitor of Intestinal Lipid Digestion and Absorption. BMC Complement. Altern. Med. 2019, 19, 242.
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus Fruit Species as a Rich Source of Bioactive Compounds. J. Food Sci. 2016, 81, C1928–C1937.
- Marchelak, A.; Olszewska, M.A.; Owczarek, A. Simultaneous Quantification of Thirty Polyphenols in Blackthorn Flowers and Dry Extracts Prepared Thereof: HPLC-PDA Method Development and Validation for Quality Control. J. Pharm. Biomed. Anal. 2020, 184, 113121.
- Owczarek, A.; Magiera, A.; Matczak, M.; Piotrowska, D.G.; Olszewska, M.A.; Marchelak, A. Optimisation of Preparative HPLC Separation of Four Isomeric Kaempferol Diglycosides from Prunus spinosa L. by Application of the Response Surface Methodology. Phytochem. Lett. 2017, 20, 415–424.
- Xu, F.; Matsuda, H.; Hata, H.; Sugawara, K.; Nakamura, S.; Yoshikawa, M. Structures of New Flavonoids and Benzofuran-Type Stilbene and Degranulation Inhibitors of Rat Basophilic Leukemia Cells from the Brazilian Herbal Medicine Cissus Sicyoides. Chem. Pharm. Bull. 2009, 57, 1089–1095.
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory Processes in Cardiovascular Disease: A Route to Targeted Therapies. Nat. Rev. Cardiol. 2017, 14, 133–144.
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant Derived and Dietary Phenolic Antioxidants: Anticancer Properties. Food Chem. 2015, 183, 235–258.
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162.
- Kovacic, P.; Somanathan, R. Cell Signaling and Receptors with Resorcinols and Flavonoids: Redox, Reactive Oxygen Species, and Physiological Effects. J. Recept. Signal Transduct. 2011, 31, 265–270.
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The Redox Chemistry of Neurodegenerative Diseases. Eur. J. Med. Chem. 2017, 133, 379–402.
- Miguel, M.G. Anthocyanins: Antioxidant and/or Anti-Inflammatory Activities. J. Appl. Pharm. Sci. 2011, 1, 7–15.
- Balta, I.; Sevastre, B.; Mireşan, V.; Taulescu, M.; Raducu, C.; Longodor, A.L.; Marchiş, Z.; Mariş, C.S.; Coroian, A. Protective Effect of Blackthorn Fruits (Prunus spinosa) against Tartrazine Toxicity Development in Albino Wistar Rats. BMC Chem. 2019, 13, 104.
- Morita, M.; Naito, Y.; Niki, E.; Yoshikawa, T. Antioxidant Action of Fermented Grain Food Supplement: Scavenging of Peroxyl Radicals and Inhibition of Plasma Lipid Oxidation Induced by Multiple Oxidants. Food Chem. 2017, 237, 574–580.
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061.
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012, 2012, 569486.
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-Inflammatory Enzymes Inhibition and Protective Effects against Oxidative Stress In Vitro. Front. Pharmacol. 2017, 8, 680.
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between Dietary Antioxidant Intakes and Cardiovascular Disease. Sci. Rep. 2022, 12, 1504.
- Vermeer, M.A.; Mulder, T.P.J.; Molhuizen, H.O.F. Theaflavins from Black Tea, Especially Theaflavin-3-Gallate, Reduce the Incorporation of Cholesterol into Mixed Micelles. J. Agric. Food Chem. 2008, 56, 12031–12036.
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278.
- Hao, R.; Shan, S.; Yang, D.; Zhang, H.; Sun, Y.; Li, Z. Peonidin-3-O-Glucoside from Purple Corncob Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Mitochondrial and Lysosome Functions to Reduce Oxidative Stress and Inflammation. Nutrients 2023, 15, 372.
- Adisakwattana, S.; Yibchok-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-Rutinoside Alleviates Postprandial Hyperglycemia and Its Synergism with Acarbose by Inhibition of Intestinal α-Glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41.
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168.
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and Anti-Inflammaging Ability of Prune (Prunus spinosa L.) Extract Result in Improved Wound Healing Efficacy. Antioxidants 2021, 10, 374.
- Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Pellati, F.; Patil, B.S. BetaSweet Carrot Extracts Have Antioxidant Activity and in Vitro Antiproliferative Effects against Breast Cancer Cells. J. Funct. Foods 2019, 62, 103552.
- Sabatini, L.; Fraternale, D.; Di Giacomo, B.; Mari, M.; Albertini, M.C.; Gordillo, B.; Rocchi, M.B.L.; Sisti, D.; Coppari, S.; Semprucci, F.; et al. Chemical Composition, Antioxidant, Antimicrobial and Anti-Inflammatory Activity of Prunus spinosa L. Fruit Ethanol Extract. J. Funct. Foods 2020, 67, 103885.
- Popović, B.M.; Blagojević, B.; Ždero Pavlović, R.; Mićić, N.; Bijelić, S.; Bogdanović, B.; Mišan, A.; Duarte, C.M.M.; Serra, A.T. Comparison between Polyphenol Profile and Bioactive Response in Blackthorn (Prunus spinosa L.) Genotypes from North Serbia-from Raw Data to PCA Analysis. Food Chem. 2020, 302, 125373.
- Marčetić, M.; Samardžić, S.; Ilić, T.; Božić, D.D.; Vidović, B. Phenolic Composition, Antioxidant, Anti-Enzymatic, Antimicrobial and Prebiotic Properties of Prunus spinosa L. Fruits. Foods 2022, 11, 3289.
- Sikora, E.; Bieniek, M.I.; Borczak, B. Composition and Antioxidant Properties of Fresh and Frozen Stored Blackthorn Fruits (Prunus spinosa L.). Acta Sci. Pol. Technol. Aliment. 2013, 12, 365–372.
- Velickovic, I.; Zizak, Z.; Rajcevic, N.; Ivanov, M.; Sokovic, M.; Marin, P.; Grujic, S. Examination of the Polyphenol Content and Bioactivities of Prunus spinosa L. Fruit Extracts. Arch. Biol. Sci. 2020, 72, 105–115.
- Kumarasamy, Y.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Comparative Studies on Biological Activities of Prunus padus and P. spinosa. Fitoterapia 2004, 75, 77–80.
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and Ethnomedicinal Uses of Plants in the District of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444.
- Alarcόn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and Local Food Plants in the South of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224.
- Gironés-Vilaplana, A.; Villaño, D.; Moreno, D.A.; García-Viguera, C. New Isotonic Drinks with Antioxidant and Biological Capacities from Berries (Maqui, Açaí and Blackthorn) and Lemon Juice. Int. J. Food Sci. Nutr. 2013, 64, 897–906.
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of Phenolic Compounds in Wild Fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730.
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic Acid Attenuates High-Fat Diet Fed-Streptozotocin-Induced Insulin Resistance via Partial Agonism of PPARγ in Experimental Type 2 Diabetic Rats and Enhances Glucose Uptake through Translocation and Activation of GLUT4 in PI3K/p-Akt Signaling Pathway. Eur. J. Pharmacol. 2014, 745, 201–216.
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant Activity, Polyphenol Content, and Related Compounds in Different Fruit Juices and Homogenates Prepared from 29 Different Pomegranate Accessions. J. Agric. Food Chem. 2007, 55, 9559–9570.
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse Polyphenol Components Contribute to Antioxidant Activity and Hypoglycemic Potential of Mulberry Varieties. LWT 2023, 173, 114308.
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 175909141989978.
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 255–277.
- Radovanović, B.C.; Anđelković, S.M.; Radovanović, A.B.; Anđelković, M.Z. Antioxidant and Antimicrobial Activity of Polyphenol Extracts from Wild Berry Fruits Grown in Southeast Serbia. Trop. J. Pharm. Res. 2013, 12, 813–819.
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938.
- Tossetta, G.; Marzioni, D. Targeting the NRF2/KEAP1 Pathway in Cervical and Endometrial Cancers. Eur. J. Pharmacol. 2023, 941, 175503.
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588.
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477.
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91.
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973.
- González-Burgos, E.; Gómez-Serranillos, M.P. Effect of Phenolic Compounds on Human Health. Nutrients 2021, 13, 3922.
