Immunotherapy has emerged as a promising new treatment modality for head and neck cancer, offering the potential for targeted and effective cancer management. Squamous cell carcinomas pose significant challenges due to their aggressive nature and limited treatment options. Conventional therapies such as surgery, radiation, and chemotherapy often have limited success rates and can have significant side effects. Immunotherapy harnesses the power of the immune system to recognize and eliminate cancer cells, and thus represents a novel approach with the potential to improve patient outcomes. In the management of head and neck squamous cell carcinoma (HNSCC), important contributions are made by immunotherapies, including adaptive cell therapy (ACT) and immune checkpoint inhibitor therapy.
1. Immune Checkpoint Inhibitor-Based Therapeutics in HNSCC
1.1. Targeting PD-1/PD-L1
The use of PD-1/PD-L1 targeting monoclonal antibodies to counteract the immunosuppressive role of PD-1 could help maintain a robust T-cell response against tumor cells
[1][99] (
Figure 12). The USA FDA has approved five monoclonal antibodies targeting PD-1 and PD-L1. Two of them target PD-L1, Atezolizumab, and Durvalumab, while Nivolumab, Pembrolizumab, and Cemiplimab are against PD-1. Nivolumab and Pembrolizumab are approved for patients with relapsed or metastatic cisplatin-resistant HNSCC
[2][100], whereas Cemiplimab exhibits significant responses in patients with metastatic cutaneous squamous cell carcinoma
[3][101].
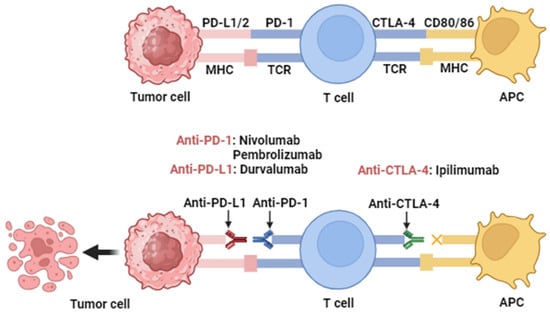
Figure 12. Action mechanisms of antibodies targeting PD-1, PD-L1, and CTLA-4. Immune checkpoint proteins PD-1 and PD-L1 are expressed in T cells, B cells, and antigen-presenting cells (APCs). High levels of PD-L1 expression hide cancer cells from T cells and enable them to grow unchecked. When PD-1 binds to PD-L1, T cells stop dividing and killing cancer cells. Through activation of PD-1/PD-L1 signaling, cancer cells evade immune responses. Inhibition of the PD-1/PD-L1 interaction by monoclonal antibodies overturns this process and augments activity. This allows T cells to become activated and kill cancer cells. This figure was created with
BioRender.com (accessed on 10 October 2023).
Nivolumab is an IgG4 monoclonal antibody that acts as an anti-PD-1 agent and blocks co-suppressive signals via the PD-1/PD-L1 signaling pathway. It was a significant milestone as the first FDA-approved immunotherapy for patients with HNSCC, based on the results of a Phase III clinical trial known as CheckMate 141 (NCT02105636)
[4][102]. The study involved 361 participants with recurrent HNSCC who had experienced progression 6 months after platinum chemotherapy. These individuals were divided into two groups in a 2:1 ratio, with one group receiving nivolumab and the other receiving standard systemic drug therapy with methotrexate, docetaxel, or cetuximab every two weeks. The primary endpoint of the study was overall survival (OS), while secondary objectives were objective response, progression-free survival (PFS), safety, and patient-reported quality of life
[5][19]. The study results showed that the group treated with nivolumab survived for a median of 7.5 months, while the standard treatment group achieved survival of 5.1 months. The estimated one-year survival rates for the nivolumab and standard treatment groups were approximately 36.0% and 16.6%, respectively. At 6 months, the PFS rate was 19.7% in the nivolumab group, compared with 9.9% in the standard treatment group. Furthermore, the incidence of severe adverse effects (grade III or IV) was lower in the nivolumab group (13.1%) compared to the standard treatment group (35.1%)
[4][102].
Pembrolizumab is a humanized IgG4-κ monoclonal antibody with a high affinity for PD-1. It received its first FDA approval in 2017 following results from the phase Ib cohort expansion study, KEYNOTE 012
[6][103]. Importantly, in advanced HNSCC patients, treatments with pembrolizumab have shown high survival rates (6-month OS rate: 58%; and 12-month OS rate: 38%) and no adverse event-related deaths, an outcome rarely seen with existing cytotoxic or targeted therapies
[7][104].
The comparison between pembrolizumab and standard treatment has been made in KEYNOTE 040 to validate that it prolongs the median OS to 8.4 months from 6.9 months for HNSCC patients
[8][105]. In addition, a phase III clinical trial called KEYNOTE 048 evaluated the use of pembrolizumab for the treatment of relapsed or metastatic HNSCC and reported significantly improved treatment outcomes with pembrolizumab in 2019. In an interim analysis, the combination of pembrolizumab and chemotherapy was found to result in longer OS than the combination of cetuximab and chemotherapy (13.0 months vs. 10.7 months) in the general patient population. Based on these compelling efficacy and safety results, pembrolizumab plus chemotherapy has become the primary treatment option for patients with recurrent or metastatic HNSCC. However, pembrolizumab monotherapy has become the first-line treatment for patients with recurrent or metastatic PD-L1
+ HNSCC
[9][20].
Emerging data have suggested that PD-L2 is an alternative ligand for PD-1, especially in PD-L1-negative patients. The interaction between PD-1 and PD-L2 exhibits a strong suppression of T cell receptor (TCR)-expressed proliferation and a reduction of cytokine production such as IL-4, IL-10, and INF-γ by CD4
+ T cells. Moreover, the expression and function of PD-L2 appear to be comparable to PD-L1 and both can reduce PD-1 signaling affecting T-cell proliferation
[10][106]. In addition, Yearley et al. used a unique immunohistochemistry assay to investigate the expression of PD-L2 in tumor tissues and the correlation between clinical response and PD-L2 status in tumor tissues from patients with relapsed/metastatic (R/M) HNSCC treated with pembrolizumab. The results indicated that there was a highly significant association between PD-L2 and PD-L1 in these tumors (
p < 0.0001)
[11][107]. However, PD-L2 expression was also detected in tumors without PD-L1 expression, suggesting that the predictive potential of PD-L2 is not dependent on PD-L1
[12][108].
Atezolizumab is an anti-PD-L1 monoclonal antibody that blocks PD-L1 and generates an anti-cancer immune response by blocking PD-L1
[13][109]. In 2014, it was used against HNSCC and other tumor types such as NSCLC, melanoma, gastric cancer, colorectal cancer, and pancreatic cancer to investigate safety and tolerability. This first Phase I study (PCD4989g; NCT01375842) did not follow the traditional clinical trial design
[14][110]. Later, in 2018, A. D. Colevas et. al. reported on the safety and long-term efficacy of atezolizumab as a single agent in a Phase Ia clinical trial in patients with HNSCC. This Phase Ia study involved 32 patients in whom treatment-related adverse events (TRAEs) of varying grades, including grades 1, 2, 3, and 4, were observed. There were no deaths and no grade 5 TRAEs. Overall survival was 6.0 months with good tolerability of atezolizumab
[15][111].
Cemiplimab is an IgG4 monoclonal antibody with high affinity and dynamics against PD-1. A study in 2018 has shown that cemiplimab is effective in reducing tumors in the phase I advanced cutaneous squamous cell carcinoma (CSCC) expansion cohort (NCT02383212) and the metastatic cohort of the phase II trial (NCT02760498)
[16][112]. Furthermore, cemiplimab is reported to exhibit marked anti-tumor activity with a fixed dose (350 mg intravenously every 3 weeks), and durable long-term effects (3 mg/kg intravenously every 2 weeks) in metastatic CSCC (NCT02760498)
[17][113].
Durvalumab is a monoclonal antibody that directly engages with PD-L1 with high affinity and, as a result, blocks the interaction with PD-1 and CD80. In the HAWK Phase II study, it was administered to individuals with recurrent and/or metastatic HNSCC who had PD-L1 expression in more than 25% of tumor cells after unsuccessful platinum-based chemotherapy
[18][114].
1.2. Targeting CTLA-4
Ipilimumab is a monoclonal antibody (mAb) targeting CTLA-4 (
Figure 12) that has been approved by the FDA for the treatment of metastatic melanoma. However, there are significant concerns about potential serious toxicity, including life-threatening colitis. Ongoing clinical trials are evaluating the use of ipilimumab in combination with cetuximab and intensity-modulated radiotherapy (IMRT) in patients with advanced HNSCC (NCT01860430 and NCT01935921). Concurrently, a phase I study is ongoing to evaluate the safety and optimal dosing of MGA271 (also known as enoblituzumab), a humanized mAb targeting CD276 (B7-H3), in combination with ipilimumab. This includes patients with B7-H3-expressing solid tumors, including HNSCC (NCT02381314). Another anti-CTLA4 antibody, tremelimumab, is also being studied in clinical trials in combination with the anti-PD-L1 antibody, durvalumab
[1][99]. A randomized phase II clinical trial with 267 R/M HNSCC patients showed that the combination of durvalumab and tremelimumab resulted in clinically relevant overall survival and manageable toxic effects
[19][115]. The clinical trial CheckMate 651 (NCT02823574) comprehensively demonstrated that a combination of nivolumab and ipilimumab is an excellent disease control agent in R/M HNSCC, increasing the median OS from 13.5 to 13.9 months compared to the EXTREME therapy
[20][116].
1.3. Targeting GITR
AMG 228 is a human IgG1 monoclonal antibody that acts as an agonist by binding to human GITR, a molecule expressed by regulatory T cells. In a phase I study (NCT02437916), 30 patients were selected with advanced solid tumors, including colorectal cancer, HNSCC, urothelial transitional cell carcinoma, non-small cell lung cancer (NSCLC), and melanoma. They administered AMG 228 intravenously every 3 weeks. By employing a two-stage dose escalation strategy: first, a single patient cohort received 3, 9, 30, or 90 mg, followed by a “rolling 6” design involving two to six patients till the maximum tolerated dose or an elevated planned dose of 1200 mg was reached. The primitive focus of the study was to estimate the safety, pharmacokinetics, pharmacodynamics, and maximum tolerated dose (MTD) based on the patients’ responses. The outcomes of the study concluded that within this patient population, AMG 228 was well tolerated up to 1200 mg. No dose-limiting toxicity (DLT) was observed, and the highest administered dose did not reach the maximum tolerated dose (MTD). However, there was no observable evidence of T-cell activation or any antitumor activity following a single administration of AMG 228 therapy
[21][117].
2. Combined Immune Checkpoint Inhibitor Therapy in Oral Cancer
2.1. PD-1/CTLA-4 Combination
The immune checkpoint inhibitors that have been used alone/combined or in combination with other therapies are listed in
Table 1. In a documented clinical case report, an unprecedented approach involving the combination of the anti-PD-1 antibody nivolumab and the anti-CTLA4 antibody ipilimumab was administered to a surgical patient diagnosed with HNSCC. Following, a 3-week course of treatment with these combined drugs, a computed tomography examination revealed a positive response in the patient’s cancer condition. However, subsequent magnetic resonance imaging (MRI) showed local recurrence at 7 months after the combined treatment. Notably, the expression levels of PD-L1 and CTLA-4 exhibited significant decreases at the cervical lymph node, whereas no substantial alteration was observed in the expression level of PD-L2 or the quantities of various immune cells, including B-cells, T-cells, T helper (Th) cells, cytotoxic and regulatory T lymphocytes, and NK cells
[22][118]. This case report suggests that combined PD-1/CTLA-4 immune checkpoint inhibitor therapy may be effective in some patients with HNSCC, but further research is needed to confirm its efficacy and safety. More research is also needed to understand why the patient’s cancer recurred despite decreases in the expression levels of PD-L1 and CTLA-4.
Table 1.
List of completed ICIs clinical trials for the treatment of head and neck cancers.
| Serial |
ICIs |
Target |
Phase |
Arm/Group |
No. of Patients |
Combination |
Clinical Trial Number |
Primary Endpoint |
Study Start/Date |
| 1 |
Durvalumab |
PD-L1 |
I |
Recurrent and/or metastatic head and neck squamous cell carcinoma |
71 |
Tremelimumab |
NCT02262741 |
Safety, tolerability, antitumor activity, PK, pharmacodynamics, and immunogenicity |
15/10/2014 |
| 2 |
Pembrolizumab |
PD-1 |
II |
Recurrent and/or metastatic head and neck squamous cell carcinoma |
172 |
After platinum-based and cetuximab therapy |
NCT02255097 |
Objective response rate (ORR) |
24/10/2014 |
| 3 |
Durvalumab |
PD-L1 |
I/II |
Head and neck cancer solid tumors |
176 |
Epacadostat |
NCT02318277 |
Safety, tolerability, pharmacokinetics, immunogenicity, and preliminary efficacy |
05/01/2015 |
| 4 |
Pembrolizumab |
PD-1 |
III |
First-line treatment of recurrent and/or metastatic head and neck squamous cell carcinoma |
882 |
Chemotherapy |
NCT02358031 |
Progression-free survival (PFS) |
19/03/2015 |
| 5 |
Pembrolizumab |
PD-1 |
II |
Advanced head and neck squamous cell carcinoma |
78 |
Acalabrutinib |
NCT02454179 |
Overall response rate (ORR) |
01/05/2015 |
| 6 |
Nivolumab |
PD-1 |
II |
Metastatic head and neck squamous cell carcinoma (HNSCC) |
65 |
Stereotactic body radiotherapy (SBRT) |
NCT02684253 |
Best overall response (BOR) |
11/02/2016 |
| 7 |
Pembrolizumab |
PD-1 |
I/II |
Locally advanced laryngeal squamous cell carcinoma (Grade 3/4) |
9 |
Chemotherapy |
NCT02759575 |
Adverse effects |
01/04/2016 |
| 8 |
Pembrolizumab |
PD-1 |
I |
Recurrent and/or metastatic head and neck squamous cell carcinoma |
36 |
Talimogene laherparepvec |
NCT02626000 |
Safety and toxicity |
06/04/2016 |
| 9 |
Nivolumab |
PD-1 |
I |
Intermediate and high-risk local regionally advanced head and neck cancer |
40 |
Chemotherapy |
NCT02764593 |
Safety |
01/06/2016 |
| 10 |
Nivolumab |
PD-1 |
II |
Recurrent or metastatic squamous cell carcinoma |
425 |
Ipilimumab |
NCT02823574 |
Overall response rate (ORR) |
08/11/2016 |
| 11 |
Nivolumab |
PD-1 |
I/II |
Neoadjuvant to surgery in advanced stage head and neck squamous cell carcinoma (HNSCC) |
33 |
Ipilimumab |
NCT03003637 |
Feasibility and safety |
28/02/2017 |
| 12 |
Nivolumab |
PD-1 |
II |
Recurrent or metastatic salivary gland carcinoma |
98 |
- |
NCT03132038 |
Progression-free survival (PFS): after 6 months of treatment |
24/03/2017 |
| 13 |
Nivolumab |
PD-1 |
III |
Recurrent and/or metastatic head and neck squamous cell carcinoma |
124 |
- |
NCT05802290 |
Adverse effects |
27/11/2017 |
| 14 |
Nivolumab |
PD-1 |
III |
Locally advanced squamous cell carcinoma |
74 |
Cisplatin in combination with radiotherapy |
NCT03349710 |
Event-free survival (EFS) |
15/12/2017 |
| 15 |
Pembrolizumab |
PD-1 |
II |
Recurrent and/or metastatic head and neck squamous cell carcinoma |
29 |
Afatinib |
NCT03695510 |
Toxicities and efficacies |
24/01/2019 |
| 16 |
Durvalumab |
PD-L1 |
I/II |
Head and neck squamous cell carcinoma |
33 |
- |
NCT03829007 |
Treatment regimen |
15/04/2019 |
| 17 |
Pembrolizumab |
PD-1 |
II |
First-line treatment of metastatic or unresectable, recurrent head and neck squamous cell carcinoma |
18 |
Ulevostinag |
NCT04220866 |
Safety and efficacy |
04/03/2020 |
| 18 |
Ipilimumab |
CTLA-4 |
I |
Head and neck cancer (Stage III-IVB) |
19 |
Cetuximab with intensity-modulated radiation therapy |
NCT01935921 |
Side effects and dosage regimen |
09/04/2013 |
| 19 |
Ipilimumab |
CTLA-4 |
I |
Head and neck squamous cell carcinoma |
80 |
Pembrolizumab |
NCT01986426 |
Safety, tolerability, PK, and efficacy |
01/11/2013 |
| 20 |
Ipilimumab |
CTLA-4 |
I/II |
Advancedstage head and neck squamous cell carcinoma |
33 |
Nivolumab (neoadjuvant to surgery) |
NCT03003637 |
Feasibility and safety |
28/02/2017 |
| 21 |
Ipilimumab |
CTLA-4 |
I |
Head and neck cancer (Stage IVA-B) |
24 |
Nivolumab with radiotherapy |
NCT03162731 |
Side effects |
11/05/2017 |
2.2. PD-1/GITR Combination
Clinical studies have shown that the combination of anti-GITR and anti-PD-1 antibody therapies can enhance the antitumor activity of T-cells. Among the anti-GITR antibodies, BMS-986156 is an agonistic human IgG1 monoclonal antibody that activates effector T cells and may also reduce or inactivate Tregs
[23][119]. A phase I/IIa clinical trial (NCT02598960) evaluated the safety and efficacy of BMS-986156 alone and in combination with the anti-PD-1 antibody, nivolumab, in patients with advanced solid tumors. The trial involved a dose-escalation design, with 66 patients receiving BMS-986156 (10–800 mg) or BMS-986156 (30–800 mg) plus nivolumab (240 mg) every 2 weeks. The study results showed that BMS-986156 ± nivolumab was well tolerated by patients and no dose-limiting toxicity occurred. Significant antitumor activity was seen when BMS-986156 was combined with nivolumab at doses predicted to have biological activity
[24][120].
3. Immune Checkpoint Inhibitors in Combination with Other Therapies
3.1. Radiotherapy
Radiotherapy (RT) is widely used to treat solid tumors, with more than 50% of patients receiving this modality. RT has the advantage over chemotherapy in minimizing systemic toxicity
[25][121]. When RT is combined with immune checkpoint inhibitors (ICIs), it can potentiate the synergistic effects, where RT contributes to the normalization of the tumor vascular system, enhance the expression of leukocyte adhesion molecules on endothelial cells, and stimulate the secretion of chemokines that attract CD8
+ T cells
[26][122]. ICI treatment is intended for recurrent or metastatic HNSCC that has previously responded to RT, especially for those whose immune cells are being transformed into immunosuppressive and radio-resistant phenotypes. In a phase II study (NCT02641093), the employment of pembrolizumab as adjuvant RT has been shown to improve the survival of patients with locally advanced HNSCC
[27][123]. In some patients, following RT can prompt somatic mutations, which can lead to the development of new tumor-associated antigens (TAAs) that can be targeted for more robust immune responses. The concurrent administration of RT and ICIs such as nivolumab (NCT02684253 and NCT03349710) is generally safe with no significant immune-related adverse events on HNSCC
[28][124].
3.2. Chemotherapy
Tumor-related antibodies are currently used primarily in combination with chemotherapy due to their limited efficacy as monotherapies
[29][125]. Chemotherapy can potentiate the efficacy of immune checkpoint inhibitor (ICI) therapy by promoting the release of neoantigens, modifying the TME through the depletion of Tregs and MDSCs, and reducing PD-L2 expression on DCs and tumor cells. Additionally, chemotherapy (CT) can stimulate APC maturation and increase MHC-I expression
[30][126]. One example of an approved combination regimen is the use of pemetrexed and carboplatin chemotherapy with pembrolizumab, where pembrolizumab exhibits minimal overlapping toxicity with the chemotherapeutic agents
[31][127]. Other combination regimens under investigation include the use of ipilimumab, paclitaxel, and carboplatin in stage-IV non-small-cell lung carcinoma (NCT02279732)
[32][67] and nivolumab with ipilimumab as the standard of care for the first-line treatment of HNSCC (NCT02741570)
[33][128]. However, it is important to note that chemotherapy can induce side effects that may interfere with the action mechanisms of ICIs, such as by inhibiting the clonal expansion of effector lymphocytes. Therefore, the potential impact of chemotherapy must be carefully assessed when designing treatment strategies.