Schwann cells, the most abundant glial cells of the peripheral nervous system, represent the key players able to supply extracellular microenvironment for axonal regrowth and restoration of myelin sheaths on regenerating axons. Following nerve injury, Schwann cells respond adaptively to damage by acquiring a new phenotype. In particular, some of them localize in the distal stump to form the Bungner band, a regeneration track in the distal site of the injured nerve, whereas others produce cytokines involved in recruitment of macrophages infiltrating into the nerve damaged area for axonal and myelin debris clearance. Several neurotrophic factors, including pituitary adenylyl cyclase-activating peptide (PACAP), promote survival and axonal elongation of injured neurons.
- peripheral nervous system
- Schwann cells
- PACAP
- neuroprotection
- regeneration
1. Introduction
2. Schwann Cells and Nerve Injury
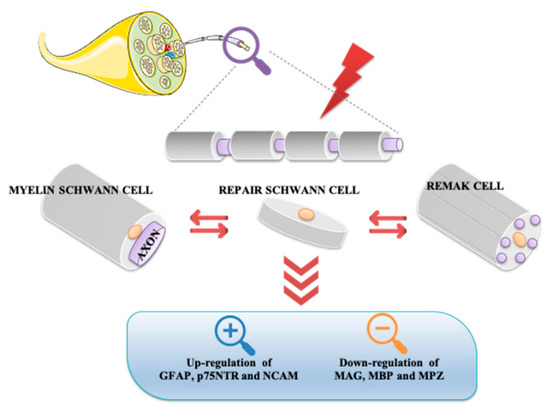
Neurons and glial cells represent the major cell types constituting the nervous system. Although in the past, glia was considered only a passive component in brain functionality, today this opinion has completely changed. Based on various evidence, it has been demonstrated that glial cells actively regulate neuronal properties and activities [4]. On the base of their morphological, biological, and functional characteristics, glial cells are classified into oligodendrocytes, microglia, ependimocytes, and astrocytes, located in the CNS, and Schwann and satellite glial cells, sited in the PNS. Schwann cells, originating from the neural crest, are the most abundant glial cells of the PNS. It is not possible to consider these cells solely as passive insulators of axons, but rather they are co-stars in the regulation of neuronal biology by providing metabolic support and regulating the response to tissue injury [5][6][7][5,6,7]. Schwann cells can be classified into Remak cells, or non-myelin Schwann cells, and myelin Schwann cells. Remak cells envelop the small diameter axons, including those of the autonomic and sensory nervous systems. These cells ensure proper development of the PNS and play an important role in regeneration after injury. In fact, the absence of myelin confers to Remak cells the ability to stimulate axonal plasticity, growth, and sprouting. Moreover, these cells modulate pain sensitivity in peripheral sensory neuropathies. In accord, it has been demonstrated that even in absence of injury, alterations between axon-Remak cells interactions are followed by neuropathic pain [2][8][2,8]. Meanwhile, Schwann cells wrap larger axons of sensory and motor neurons [2][9][2,9]. Myelinating Schwann cells are radially and longitudinally polarized cells surrounding the axon and delimiting nodal, paranodal, juxtaparanodal, and internodal compartments [10]. Among these, the internodal compartment is the largest domain representing 99% of the myelinating Schwann cell length. Compared to Remak cells, these cells form a myelin sheath wrapping around the axon several times. Myelin is characterized by high lipid content (70%) enriched with glycosphingolipids, saturated long-chain fatty acids, and cholesterol, the latter involved in the assembly of myelin sheath. Synthesis, maintenance, and structure of the myelin sheath is also regulated by several molecules including myelin protein zero (MPZ), pro-myelin transcription factor Egr2 (Krox20), maltose-binding protein (MBP), and myelin associated glycoprotein (MAG). MPZ is a transmembrane adhesion molecule of the immunoglobulin gene superfamily, needing cholesterol for proper endoplasmic reticulum (ER) export. Krox20 controls a set of genes required for the completion of nerve myelination [11][13]. MBP is a peripheral membrane protein promoting myelin membrane stacking [12][14], whereas MAG is a membrane associated protein supporting myelin-axon stability and regulating the axon cytoskeleton [13][14][15,16]. Schwann cells exhibit great plasticity, a peculiar feature allowing them to actively adapt and convert into cells sustaining regeneration following nerve injury [15][17]. Nerve injuries can be classified by severity into three broad grades: neurapraxia, axonotmesis, and neurotmesis [16][18] (Figure 1). Neurapraxia is the mildest type of injury which does not imply loss of nerve continuity. It occurs after nerve compression or blocked blood flow to the affected nerves. It is characterized by a temporary failure to conduct signals due to a local ion-induced conduction block at the injury site or alterations in the myelin structure [17][19].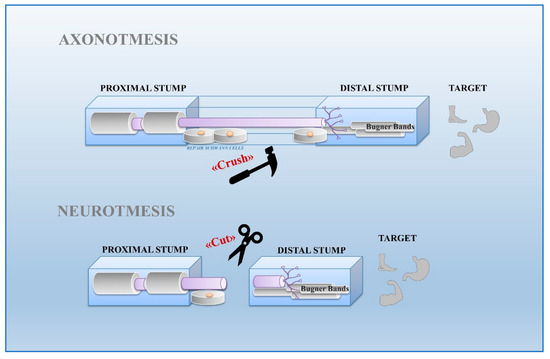
3. Effect of PACAP on Schwann Cells during Nerve Injury
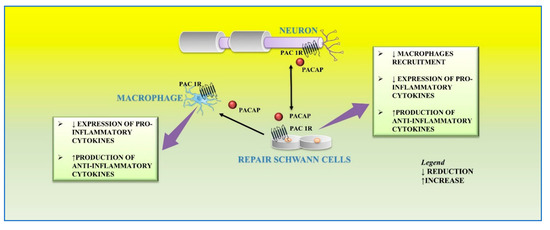
In response to peripheral nerve injury, the level of PACAP significantly increases both in sensory and motor neurons [24][25][26][83,84,85]. In particular, in the cultured rat vagus nerve, it is up regulated and detectable up to one month after nerve damage, corresponding to the period of axon regeneration [27][86]. In the site of damaged vagus nerve, PACAP up-regulation induces the stimulation of cells surrounding the regenerating fibers including Schwann cells [27][86]. Moreover, Armstrong et al. [28][87], showed that axon regeneration following injury is strongly reduced in PACAP knockout animals. Axons of lesioned neurons release PACAP in the surrounding area, which binds to its receptors localized on cultured primary rat Schwann cells and macrophages. This peptide plays different functions on the distal stump related to different phases of regeneration. After peripheral nerve injury, a large number of macrophages infiltrates into the distal nerve stump area to clear axonal and myelin debris. This event is further sustained by the release of proinflammatory cytokines from resident macrophages that promote the recruitment of other macrophages to the distal stump [29][88]. Moreover, Schwann cells are involved in their recruitment through the release of cytokines, such as TNF-a, interleukin (IL)-1a, IL-1b, and monocyte chemotactic protein 1 (MCP-1) from the distal stump [19][27]. Therefore, this inflammatory vicious cycle must be regulated in order to avoid excessive inflammation and further tissue damage. In the early step of peripheral nerve injury, PACAP plays a regulatory effect by balancing pro-inflammatory cytokine production from Schwann cells and preventing excessive macrophage recruitment. The paracrine role of PACAP in neuronal survival and axon outgrowth during peripheral nerve regeneration is related to the up-regulation of its receptors. In accord, high expression levels of PAC1, VPAC1, and VPAC2 receptors have been demonstrated in both Schwann cells and macrophages within the distal stump after mouse sciatic nerve injury [23][32]. Stimulation of PACAP receptors may rapidly activate MAPK/ERK or PI3K/Akt pathways, both involved in maintenance of myelinated peripheral nerves and proliferation of Schwann cells after injury [30][31][89,90]. Moreover, the exposure of the RT4-D6P2T Schwann cell line to an inflammatory stimulus, such as lipopolysaccharide (LPS), significantly increased not only the levels of pro-inflammatory cytokines such as IL-6, IL-18, and TNF but also PACAP release [32][91]. In the later step of regeneration, PACAP, released from neurons as well as Schwann cells, down-regulates the expression of pro-inflammatory cytokines and up-regulates the production of anti-inflammatory cytokines from macrophages by favoring the macrophage stage transition in order to end the inflammatory response in the distal nerve stump. In accord, it has been found that PACAP increases the expression of anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13, in the distal nerve explant [23][32] (Figure 3).