Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Raffaele Serra.
Chronic Kidney Disease (CKD) represents a risk factor for fatal and nonfatal cardiovascular (CV) events, including peripheral vascular disease (PVD). This occurs because CKD encompasses several factors that lead to poor prognoses, mainly due to a reduction of the estimated glomerular filtration rate (eGFR), the presence of proteinuria, and the uremic inflammatory milieu. The matrix metalloproteinases (MMPs) are a group of zinc-containing endopeptidases implicated in extracellular matrix (ECM) remodeling, a systemic process in tissue homeostasis. MMPs play an important role in cell differentiation, angiogenesis, inflammation, and vascular damage.
- metalloproteinases
- MMPs
- TIMPs
- CKD
- peripheral vascular disease
- biomarkers
- proteinuria
- eGFR
- PAD.
1. Introduction
Chronic Kidney Disease (CKD) is defined as the presence of abnormalities in kidney function or structure for at least 3 months [1]. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines classify CKD according to the level of estimated glomerular filtration rate (eGFR), a marker of kidney function, and the amount of urine protein (proteinuria or albuminuria), which represents the principal marker of kidney damage and the primary cause of CKD [2]. The onset of CKD exerts a deleterious impact on individual health. Indeed, it has been demonstrated that either an eGFR reduction < 60 mL/min/1.73 m2 or a small increase in proteinuria are associated with a poor prognosis, as shown by the increased rate of cardiovascular (CV) fatal and nonfatal events, all-cause mortality, and the progression of kidney disease resulting in the need for renal replacement therapies (kidney transplantation or dialysis) [3,4][3][4].
CV risk is severely increased in patients with CKD, and the impact of CV events in this population is crucial if one considers that the rate of CV events (such as myocardial infarction, stroke, arrhythmias, peripheral vascular disease, and chronic heart failure) over time is higher than the risk for kidney disease progression [5]. This strong association has been attributed to the coexistence of traditional and nontraditional CV risk factors in CKD patients, with the former being represented by hypertension, smoking, hypercholesterolemia, and metabolic abnormalities, and the latter by the two main prognostic measures of CKD, i.e., proteinuria and eGFR [6,7][6][7].
Among the wide spectrum of CV events, a relevant role is portrayed by peripheral vascular disease (PVD). It has been demonstrated that the presence of mild-to-moderate CKD increases the risk of peripheral artery disease, leg revascularization, leg amputation, and hospitalizations [8]. Either an eGFR reduction below 60 mL/min/1.73 m2 or slight increases of albuminuria (>30 mg/g) have been associated with a 1.5 to 4 times higher risk of peripheral artery disease (PAD). This evidence is strong, being derived from patients without PAD at basal visit, and reproducible, being confirmed in the general population, as well as in high-risk populations, regardless of the geographic area. Notably, the rate of PVD among patients with End-Stage Kidney Disease (ESKD) is higher than the incidence of acute myocardial infarction, stroke, and arrhythmias [9]. Hence, CKD patients warrant clinical surveillance and prompt the need for strategies to prevent the onset of PVD.
The magnitude of the association is so important that research has recently focused on discovering new biomarkers that are potentially useful in the clinical management of CKD patients at increased risk for PVD. Matrix Metalloproteinases (MMPs) are zinc-containing endopeptidases that are involved in regulating tissue development and homeostasis [10]. Although all MMPs are better acknowledged for their role in remodeling the extracellular matrix (ECM), they actually interact with both ECM and non-ECM substrates. Cell adhesion molecules and growth factors or their receptors represent these latter group of substrates. The wide range of interactions in which MMPs play an active role also explains why these endopeptidases participate in a number of functions such as cell differentiation, migration, apoptosis, and angiogenesis. On the other hand, MMPs have also been attributed to a profibrotic and pro-inflammatory role. MMP-2 and MMP-7 were increased in plasma and urine samples of CKD patients, and may affect direct damage to the kidney with the onset of albuminuria [11]. Moreover, imbalances in the expression and the levels of MMPs or their inhibitors have been linked to the structural changes that occur in the development of PAD, atherosclerotic plaque maturation, and arterial remodeling [12].
2. Metalloproteinases and the Kidney
MMPs are classified, according to their structure (or function) and the substrate selectivity, into six groups: Collagenases (MMP-1, MMP-8 and MMP-13), which cleave native collagen and with possible antifibrotic function; Gelatinases (MMP-2 and MMP-9), whose function is to cleave denatured collagens, type IV collagens in basement membranes and some chemokines; Stromelysins (MMP-3, MMP-10, MMP-11 and MMP-19), which degrade a number of substances such as fibronectin, laminin but are unable to cleave native collagen; Matrilysins (MMP-7 and MMP-26) act by degrading ECM components (laminin and entactin); Membrane-type MMPs (MMP-14, -15, -16, -17, -24, and -25), so called because they are anchored to the exterior of the cell membrane; and other MMPs that are tissue or cell-type specific [10,13][10][13]. The MMP activity is modulated by a series of four known enzymes called tissue inhibitors of metalloproteinases (TIMPs). TIMPs participate either in the activation or inhibition of MMP activity and, like MMPs, regulate several cellular functions such as cell proliferation, apoptosis, and angiogenesis [14]. It has been demonstrated that MMPs exert a role in the development of proteinuric kidney diseases in humans. Indeed, a number of studies depicted increased serum and urine levels of MMP-2, -8, and -9 in diabetic patients, with MMP-9 in particular being positively correlated with the degree of proteinuria in these patients [15,16,17,18][15][16][17][18]. It is remarkable that, in addition to MMP-9, urinary levels of neutrophil gelatinase-associated lipocalin (NGAL) have been found to increase in patients with diabetic nephropathy [19]. NGAL and MMP-9 are coexpressed, and their interaction prevents the degradation of MMP-9. It has, thus, been postulated that the increase in NGAL may prolong the action of MMP-9 as a trigger of kidney damage [20]. MMP-7, which is normally expressed in the proximal and distal convoluted tubules, as well as in the collecting duct, is found to be overexpressed in diabetic patients where it is also inversely correlated with the degree of kidney function [21]. Other than in diabetic patients, MMP expression is altered in many other glomerular diseases. Typical patterns of MMP-2 and MMP-9 are differentially expressed in patients with focal segmental glomerulosclerosis, minimal change disease, membranous nephropathy, and ANCA-associated vasculitis [22,23,24,25,26][22][23][24][25][26]. Regardless of the specific kidney disease involved, several mechanisms of damage have been put forward to explain the pathophysiologic effects of MMPs (Figure 1).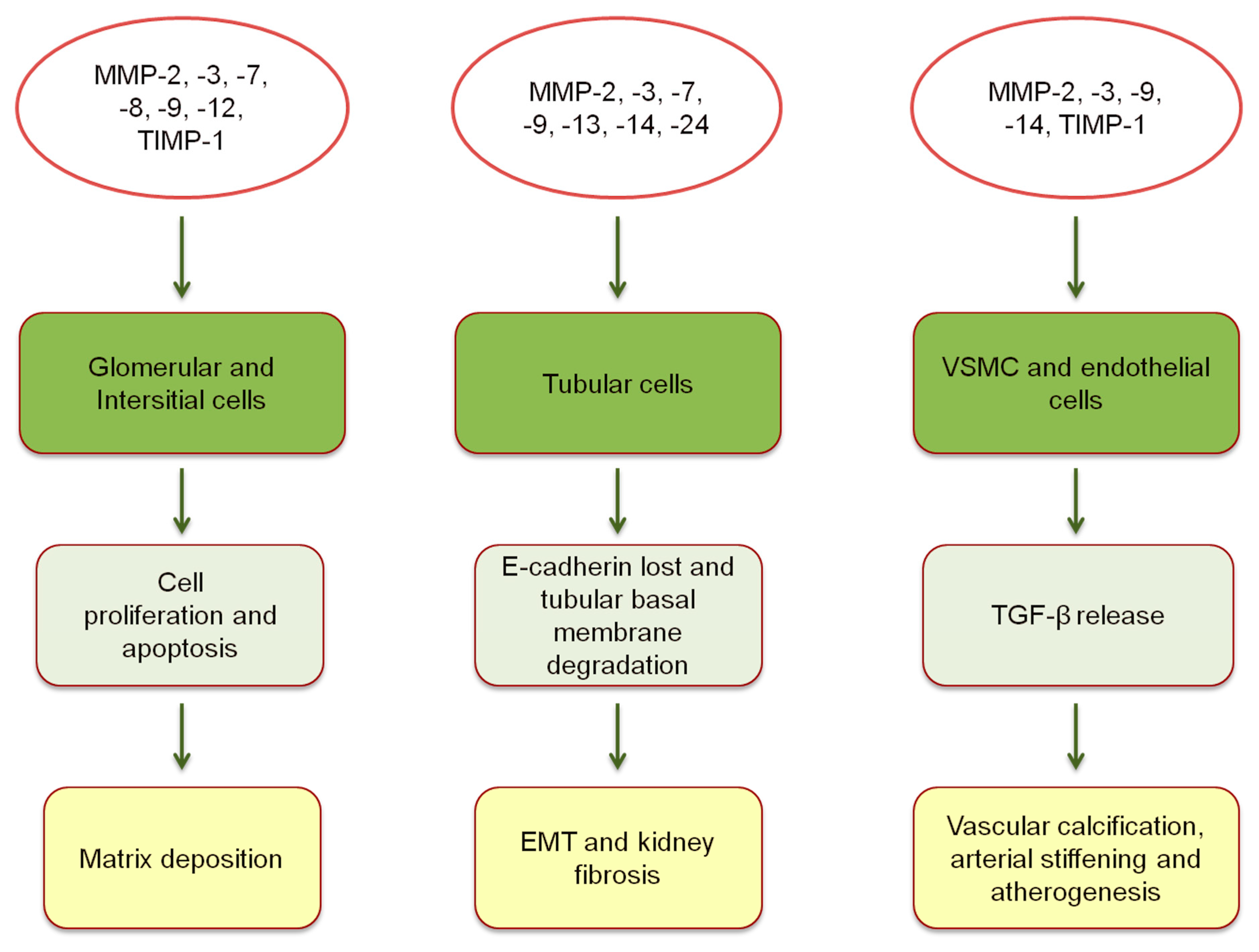
3. Vascular Effects of Metalloproteinases in CKD Patients
The structural remodeling of ECM, together with the profibrotic effect of MMPs, are detrimental for other organs apart from the kidney. Elevated blood concentrations of TIMP-1 have been associated with an increased risk of developing chronic heart failure (CHF) and, in patients already diagnosed with CHF, they were predictors of poor prognoses [41,42][41][42]. Moreover, the increase in MMP-9 and TIMP-1 conferred risk of all-cause mortality and incident CV disease in community studies [43,44][43][44]. Possible explanations for the relationship between MMPs and CV risk are varied. Whereas all MMPs are likely risk factors for atherosclerosis and cardiac dysfunction, a more specific mechanism of damage has been postulated for MMP-9 and TIMP-1. Indeed, MMP-9 is involved in the intracellular cleavage of myosin filaments, a mechanism that leads to ventricular hypertrophy [45]. TIMP-1 has shown a direct relation with the left ventricular mass in the Framingham Heart Study participants [41]. The CKD condition is associated with an increased prevalence of CV morbidity and mortality. The United States Renal Data System (USRDS) showed that the frequency of each CVD, including myocardial infarction, coronary artery disease, and peripheral vascular disease was higher among patients with CKD compared with those without (Figure 2) [9].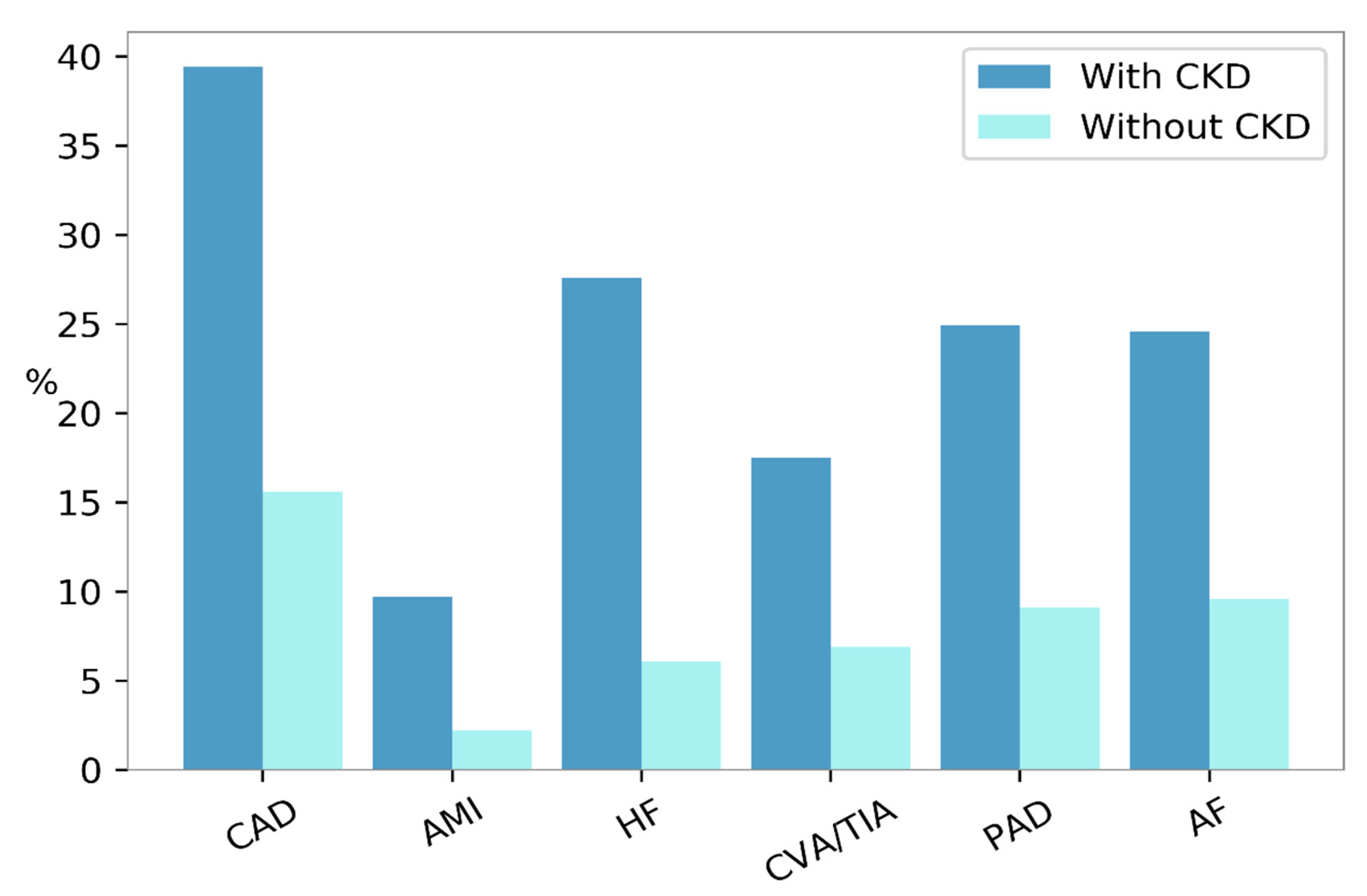
Figure 2. Prevalence of cardiovascular diseases according to the presence (dark blue bars) or absence (turquoise bars) of Chronic Kidney Disease (CKD) in the United States, in the year 2015. AF, atrial fibrillation; AMI, acute myocardial infarction; CAD, coronary artery disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; HF, heart failure; PAD, peripheral arterial disease.
4. Metalloproteinases and Peripheral Vascular Disease
MMPs play a role in the pathogenesis and prognosis of arterial and venous disease. With respect to the damage occurring in the arterial tissue, multiple studies have shown that MMPs are involved in one or more steps of atherogenesis and aneurysm development [58]. Evidence that confirms this hypothesis comes from both basic and clinical studies. During the progression of atherosclerotic plaques, a number of MMPs are produced, including TIMP-1, MMP-1, -2, -3, -9, and -14 [59]. An in vivo study on Fischer male rats showed that TIMP-1 directly regulates the smooth muscle cell migration [27]. A similar function has been recognized in human studies on MMP-14 and TIMP-1, with being both involved in the process of cellular migration to the plaque fibrous cap and plaque inflammation [28,29][28][29]. MMP-9 also contributes to the destruction of the fibrous cap itself in patients with increased CV risk [30]. Interestingly, increased concentrations of MMP-2, -3, and -9 have been found within the aneurysm human tissue, being mainly produced by the macrophages localized in the aneurysm wall [31]. These different enzymes are differentially expressed according to the aneurysm dimensions and severity [60,61][60][61]. MMP-2 is increased in small aneurysms (<5.5 cm), whereas MMP-9 is dominant in large aneurysms (5.5–7 cm). Moreover, it has also been shown that different localizations of MMP activity within the aneurysm wall modify the risk of rupture [60]. In clinical studies, abnormal circulating levels of MMP-1, -2, -8, -9, and TIMP-1 were found in patients with Peripheral Arterial Disease; their increase was attributed to the presence of ischemic tissue [12,62,63][12][62][63]. Circulating levels of MMP-1 and -8 were also found as predictors of poor prognosis, in terms of major amputation or death, in patients who underwent lower extremity bypass [64]. These multiple findings enhanced the importance of MMPs as biomarkers of arterial disease severity that also provide important prognostic information in clinical practice. Regarding venous diseases, it has been observed that alterations in ECM remodeling are common in the case of varicose veins and chronic venous insufficiency (CVI). With regards to varicose veins, the expression of MMP-1, -2, -3, -7, and -9 is increased, particularly in the smooth muscular cell of the vein wall, both in human and mice models [65,66][65][66]. Moreover, an analysis of human saphenous vein showed that this expression is even higher in varicose veins with inflammation, as compared to those without inflammation [65]. Mechanisms underlying the association between MMPs and varicose vein physiopathology might involve the effect of MMPs on ECM degradation and the relaxation of the venous wall [66]. An upregulation of MMP-1, -2, -9, and -13, together with a downregulation of TIMP-1 and TIMP-2, have been described in patients with CVI [67,68][67][68]. The distribution of MMP varies based upon the stage (from CVI to active wound) and cells, suggesting that MMP-1 and TIMP-1 are needed in the re-epithelialization phase, while MMP-9 and -13 primarily participate in the remodeling of the collagenous matrix [69].5. Synthetic Metalloproteinases Inhibitors in Experimental and Clinical Research
MMP activity is regulated at different levels, including either intracellular (mRNA expression and post-translational modification of MMP structure) or extracellular (stimulation or inhibition of their enzymatic activity from endogenous or exogenous substrates) process. The net activity of MMPs is a crucial step, since its up- or down- regulation could affect MMP activity, and ultimately lead to metabolic diseases, cancer, cardiovascular, and renal disorders [72][70]. For these reasons, new pharmacological agents that interfere with MMP activity have been developed and utilized as potential tools that could benefit a wide spectrum of patients. Synthetic MMPs inhibitors (MMPs-I) include broad-spectrum and specific MMPs-I. The vast majority of these compounds contain Zn2+ in their structure, and have structured as Zn2+ binding globulin (ZBG). Indeed, ZBGs inactivate MMPs by displacing the Zn2+- bound water in the MMPs, and favor the anchorage of the drug to the MMPs substrate binding-pocket [73][71]. ZBGs encompass hydroxamic acids Batimastat (BB-94), Marimastat (BB-2516), and Ilomastat (GM6001), that displays a broad-spectrum inhibition of MMPs. More selective ZBGs molecules have also been developed, and include hydrazides and sulfonylhydrazides that specifically inhibit MMP-1, -2, and -9. Hydroxamic ZBGs are effective, but they have poor oral bioavailability and, by inhibiting multiple MMPs, cause several musculoskeletal side effects [74][72]. Hence, heterocyclic bidentate chelators have been developed that have shown more biostability and lower toxicity in cells assays. Tetracyclines are antibiotic molecules that, by chelating Zn2+ ion, are able to inhibit MMPs. Doxycycline inhibits MMP-2 and-9 [75][73]. Chemically-modified tetracyclines reach higher plasma levels for prolonged periods of time, require less frequent administration, are associated with lower rate of side effects when administered orally, and are thus preferred over conventional tetracyclines [76][74]. Apart from zinc-based compounds, several MMPs-I act by a noncompetitive, nonzinc-binding, mechanism of inhibition. They show high selectivity that minimizes the side effects, and thus, are considered very promising molecules [77][75]. MMPs-Is have already been used in pilot studies in mice and in human models of kidney damage [11]. MMPs-I BB-1101 has shown to reduce proteinuria in rats with anti-Thy1.1 nephritis, an experimental model of glomerular damage induced by antibody against Thy 1 gene [78][76]. A similar effect on proteinuria was found with the MMPs-I BB-94 in an experimental model of kidney allograft rejection in mice and the tetracycline antibiotic doxycycline in human patients with diabetic nephropathy already under renin-angiotensin-aldosterone inhibition [79,80][77][78]. Interestingly, doxycycline also reduced the aneurysm expansion in small randomized clinical trials enrolling patients with abdominal aortic aneurysm [81,82][79][80].References
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013, 3 (Suppl. 2013), 1–150.
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081.
- Gansevoort, R.T.; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011, 80, 93–104.
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2008, 351, 1296–1305, Erratum in N. Engl. J. Med. 2008, 18, 4.
- De Nicola, L.; Minutolo, R.; Chiodini, P.; Zoccali, C.; Castellino, P.; Donadio, C.; Strippoli, M.; Casino, F.; Giannattasio, M.; Petrarulo, F.; et al. Global approach to cardiovascular risk in chronic kidney disease: Reality and opportunities for intervention. Kidney Int. 2006, 69, 538–545.
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525.
- Matsushita, K.; Ballew, S.H.; Coresh, J.; Arima, H.; Ärnlöv, J.; Cirillo, M.; Ebert, N.; Hiramoto, J.S.; Kimm, H.; Shlipak, M.G.; et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017, 5, 718–728.
- United States Renal Data System. 2017. Available online: https://www.usrds.org/2017/view/Default.aspx (accessed on 6 November 2019).
- Tan, R.J.; Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 2012, 302, F1351–F1361.
- Parrish, A.R. Matrix Metalloproteinases in Kidney Disease: Role in Pathogenesis and Potential as a Therapeutic Target. Prog. Mol. Biol. Transl. Sci. 2017, 148, 31–65.
- Tayebjee, M.H.; Tan, K.T.; Mac Fadyen, R.J.; Lip, G.Y. Abnormal circulating levels of metalloprotease 9 and its tissue inhibitor 1 in angiographically proven peripheral arterial disease: Relationship to disease severity. J. Intern. Med. 2005, 257, 110–116.
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal Physiol. 2007, 292, F905–F911.
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Gharagozlian, S.; Svennevig, K.; Bangstad, H.J.; Winberg, J.O.; Kolset, S.O. Matrix metalloproteinases in subjects with type 1 diabetes. BMC Clin. Pathol. 2009, 9, 7:1–7:5.
- Lauhio, A.; Sorsa, T.; Srinivas, R.; Stenman, M.; Tervahartiala, T.; Stenman, U.H.; Gronhagen-Riska, C.; Honkanen, E. Urinary matrix metallo-proteinase-8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann. Med. 2008, 40, 312–320.
- Li, Y.; Kang, Y.S.; Dai, C.; Kiss, L.P.; Wen, X.; Liu, Y. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 2008, 172, 299–308.
- Tashiro, K.; Koyanagi, I.; Ohara, I.; Ito, T.; Saitoh, A.; Horikoshi, S.; Tomino, Y. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2004, 18, 206–210.
- Thrailkill, K.M.; Moreau, C.S.; Cockrell, G.E.; Jo, C.H.; Bunn, R.C.; Morales-Pozzo, A.E.; Lumpkin, C.K.; Fowlkes, J.L. Disease and gender-specific dysregulation of NGAL and MMP-9 in type 1 diabetes mellitus. Endocrine 2010, 37, 336–343.
- Tschesche, H.; Zolzer, V.; Triebel, S.; Bartsch, S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur. J. Biochem. 2001, 268, 1918–1928.
- Ban, C.R.; Twigg, S.M.; Franjic, B.; Brooks, B.A.; Celermajer, D.; Yue, D.K.; McLennan, S.V. Serum MMP-7 is increased in diabetic renal disease and diabetic diastolic dysfunction. Diabetes Res. Clin. Pract. 2010, 87, 335–341.
- Huja, T.S.; Gopalani, A.; Davies, P.; Ahuja, H. Matrix metalloproteinase-9 expression in renal biopsies of patients with HIV-associated nephropathy. Nephron. Clin. Pract. 2003, 95, c100–c104.
- Bauvois, B.; Mothu, N.; Nguyen, J.; Nguyen-Khoa, T.; Noel, L.H.; Jungers, P. Specific changes in plasma concentrations of matrix metalloproteinase-2 and -9, TIMP-1 and TGF-beta1 in patients with distinct types of primary glomerulonephritis. Nephrol. Dial. Transplant. 2007, 22, 1115–1122.
- Czech, K.A.; Bennett, M.; Devarajan, P. Distinct metalloproteinase excretion patterns in focal segmental glomerulosclerosis. Pediatr. Nephrol. 2011, 26, 2179–2184.
- Sanders, J.S.; Huitema, M.G.; Hanemaaijer, R.; van Goor, H.; Kallenberg, C.G.; Stegeman, C.A. Urinary matrix metalloproteinases reflect renal damage in anti-neutrophil cytoplasm autoantibody-associated vasculitis. Am. J. Physiol. Renal Physiol. 2007, 293, F1927–F1934.
- Sanders, J.S.; van Goor, H.; Hanemaaijer, R.; Kallenberg, C.G.; Stegeman, C.A. Renal expression of matrix metalloproteinases in human ANCA- associated glomerulonephritis. Nephrol. Dial. Transplant. 2004, 19, 1412–1419.
- Forough, R.; Koyama, N.; Hasenstab, D.; Lea, H.; Clowes, M.; Nikkari, S.T.; Clowes, A.W. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ. Res. 1996, 79, 812–820.
- Sritharan, K.; Essex, D.; Sandison, A.; Ellis, M.; Monaco, C.; Davies, A.H. Membrane Type-1 matrix metalloproteinase: A key player in carotid plaque instability and symptomatic carotid atherosclerotic disease. Br. J. Surg. 2008, 95, 2.
- Sritharan, K.; Navin, T.; Ellis, M.; Monaco, C.; Davies, A.H. Membrane type-1 matrix metalloproteinase is upregulated in symptomatic carotid atherosclerotic disease and along with other members of the quartenary complex regulated by pro-inflammatory cytokines. Br. J. Surg. 2008, 95, 934.
- Blankenberg, S.; Rupprecht, H.J.; Poirier, O.; Bickel, C.; Smieja, M.; Hafner, G.; Meyer, J.; Cambien, F.; Tiret, L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 2003, 107, 1579–1585.
- Newman, K.M.; Jean-Claude, J.; Li, H.; Scholes, J.V.; Ogata, Y.; Nagase, H.; Tilson, M.D. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J. Vasc. Surg. 1994, 20, 814–820.
- Henger, A.; Kretzler, M.; Doran, P.; Bonrouhi, M.; Schmid, H.; Kiss, E.; Cohen, C.D.; Madden, S.; Porubsky, S.; Gröne, E.F.; et al. Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression. Kidney Int. 2004, 65, 904–917.
- Hu, Y.; Ivashkiv, L.B. Costimulation of chemokine receptor signaling by matrix metalloproteinase-9 mediates enhanced migration of IFN-alpha dendritic cells. J. Immunol. 2006, 176, 6022–6033.
- Li, Q.; Park, P.W.; Wilson, C.L.; Parks, W.C. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002, 111, 635–646.
- Essick, E.; Sithu, S.; Dean, W.; D’Souza, S. Pervanadate-induced shedding of the intercellular adhesion molecule (ICAM)-1 ectodomain is mediated by membrane type-1 matrix metalloproteinase (MT1-MMP). Mol. Cell Biochem. 2008, 314, 151–159.
- Yamashita, C.M.; Dolgonos, L.; Zemans, R.L.; Young, S.K.; Robertson, J.; Briones, N.; Suzuki, T.; Campbell, M.N.; Gauldie, J.; Radisky, D.C.; et al. Matrix metalloproteinase 3 is a mediator of pulmonary fibrosis. Am. J. Pathol. 2011, 179, 1733–1745.
- Gagliano, N.; Arosio, B.; Santambrogio, D.; Balestrieri, M.R.; Padoani, G.; Tagliabue, J.; Masson, S.; Vergani, C.; Annoni, G. Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B365–B372.
- Kim, H.; Oda, T.; Lopez-Guisa, J.; Wing, D.; Edwards, D.R.; Soloway, P.D.; Eddy, A.A. TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 2001, 12, 736–748.
- Lu, Y.; Liu, S.; Zhang, S.; Cai, G.; Jiang, H.; Su, H.; Li, X.; Hong, Q.; Zhang, X.; Chen, X. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol. Cells 2011, 31, 225–230.
- Lieb, W.; Song, R.J.; Xanthakis, V.; Vasan, R.S. Association of Circulating Tissue Inhibitor of Metalloproteinases-1 and Procollagen Type III Aminoterminal Peptide Levels With Incident Heart Failure and Chronic Kidney Disease. J. Am. Heart Assoc. 2019, 8, e011426.
- Sundstrom, J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Sawyer, D.B.; Siwik, D.A.; Colucci, W.S.; Wilson, P.W.; Vasan, R.S. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: The Framingham Heart Study. Eur. Heart J. 2004, 25, 1509–1516.
- Morishita, T.; Uzui, H.; Mitsuke, Y.; Amaya, N.; Kaseno, K.; Ishida, K.; Fukuoka, Y.; Ikeda, H.; Tama, N.; Yamazaki, T.; et al. Association between matrix metalloproteinase-9 and worsening heart failure events in patients with chronic heart failure. ESC Heart Fail. 2017, 4, 321–330.
- Hansson, J.; Vasan, R.S.; Arnlov, J.; Ingelsson, E.; Lind, L.; Larsson, A.; Michaelsson, K.; Sundstrom, J. Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: Cohort study. PLoS ONE 2011, 6, e16185.
- Agarwal, I.; Glazer, N.L.; Barasch, E.; Biggs, M.L.; Djousse, L.; Fitzpatrick, A.L.; Gottdiener, J.S.; Ix, J.H.; Kizer, J.R.; Rimm, E.B.; et al. Fibrosis-related biomarkers and risk of total and cause-specific mortality: The Cardiovascular Health Study. Am. J. Epidemiol. 2014, 179, 1331–1339.
- Rouet-Benzineb, P.; Buhler, J.M.; Dreyfus, P.; Delcourt, A.; Dorent, R.; Perennec, J.; Crozatier, B.; Harf, A.; Lafuma, C. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: Potential role of MMP-9 in myosin-heavy chain degradation. Eur. J. Heart Fail. 1999, 1, 337–352.
- Provenzano, M.; Mancuso, C.; Garofalo, C.; De Nicola, L.; Andreucci, M. Temporal variation of Chronic Kidney Disease’s epidemiology. G. Ital. Nefrol. 2019, 36, 2019-vol2.
- Minutolo, R.; Gabbai, F.B.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Sasso, F.C.; Santoro, D.; Bellizzi, V.; Conte, G.; et al. Cardiorenal prognosis by residual proteinuria level in diabetic chronic kidney disease: Pooled analysis of four cohort studies. Nephrol. Dial. Transplant. 2018, 33, 1942–1949.
- Matsushita, K.; Sang, Y.; Ballew, S.H.; Shlipak, M.; Katz, R.; Rosas, S.E.; Peralta, C.A.; Woodward, M.; Kramer, H.J.; Jacobs, D.R.; et al. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J. Am. Soc. Nephrol. 2015, 26, 439–447.
- Pawlak, K.; Pawlak, D.; Mysliwiec, M. Extrinsic coagulation pathway activation and metalloproteinase-2/TIMPs system are related to oxidative stress and atherosclerosis in hemodialysis patients. Thromb. Haemost. 2004, 92, 646–653.
- Pawlak, K.; Pawlak, D.; Mysliwiec, M. Urokinase-type plasminogen activator and metalloproteinase-2 are independently related to the carotid atherosclerosis in haemodialysis patients. Thromb. Res. 2008, 121, 543–548.
- Nagano, M.; Fukami, K.; Yamagishi, S.; Ueda, S.; Kaida, Y.; Matsumoto, T.; Yoshimura, J.; Hazama, T.; Takamiya, Y.; Kusumoto, T.; et al. Circulating matrix metalloproteinase-2 is an independent correlate of proteinuria in patients with chronic kidney disease. Am. J. Nephrol. 2009, 29, 109–115.
- Peiskerová, M.; Kalousová, M.; Kratochvílová, M.; Dusilová-Sulková, S.; Uhrová, J.; Bandúr, S.; Malbohan, I.M.; Zima, T.; Tesař, V. Fibroblast growth factor 23 and matrix-metalloproteinases in patients with chronic kidney disease: Are they associated with cardiovascular disease? Kidney Blood Press. Res. 2009, 32, 276–283.
- Pawlak, K.; Tankiewicz, J.; Mysliwiec, M.; Pawlak, D. Systemic levels of MMP2/TIMP2 and cardiovascular risk in CAPD patients. Nephron. Clin. Pract. 2010, 115, c251–c258.
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 384–392.
- Brunet, P.; Gondouin, B.; Duval-Sabatier, A.; Dou, L.; Cerini, C.; Dignat-George, F.; Jourde-Chiche, N.; Argiles, A.; Burtey, S. Does uremia cause vascular dysfunction? Kidney Blood Press. Res. 2011, 34, 284–290.
- Newby, A.C. Dual role of matrix metalloproteinases (matrix-ins) in intimal thickening and atherosclerotic plaque rupture. Physiol. Rev. 2005, 85, 1–31.
- Libby, P. Collagenases and cracks in the plaque. J. Clin. Investig. 2013, 123, 3201–3203.
- Lim, C.S.; Shalhoub, J.; Gohel, M.S.; Shepherd, A.C.; Davies, A.H. Matrix metalloproteinases in vascular disease—A potential therapeutic target? Curr. Vasc. Pharmacol. 2010, 8, 75–85.
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Chesire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639.
- McMillan, W.D.; Tamarina, N.A.; Cipollone, M.; Johnson, D.A.; Parker, M.A.; Pearce, W.H. Size matters: The relationship between MMP-9 expression and aortic diameter. Circulation 1997, 96, 2228–2232.
- Freestone, T.; Turner, R.J.; Coady, A.; Higman, D.J.; Greenhalgh, R.M.; Powell, J.T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1145–1151.
- Martinez-Aguilar, E.; Gomez-Rodriguez, V.; Orbe, J.; Rodriguez, J.A.; Fernández-Alonso, L.; Roncal, C.; Páramo, J.A. Matrix metalloproteinase 10 is associated with disease severity and mortality in patients with peripheral arterial disease. J. Vasc. Surg. 2015, 61, 428–435.
- Morishita, T.; Uzui, H.; Nakano, A.; Mitsuke, Y.; Geshi, T.; Ueda, T.; Lee, J.D. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metalloproteinase. J. Atheroscler. Thromb. 2012, 19, 149–158.
- De Caridi, G.; Massara, M.; Spinelli, F.; David, A.; Gangemi, S.; Fugetto, F.; Grande, R.; Butrico, L.; Stefanelli, R.; Colosimo, M.; et al. Matrix metalloproteinases and risk stratification in patients undergoing surgical revascularisation for critical limb ischaemia. Int. Wound J. 2016, 13, 493–499.
- Woodside, K.J.; Hu, M.; Burke, A.; Murakami, M.; Pounds, L.L.; Killewich, L.A.; Daller, J.A.; Hunter, G.C. Morphologic characteristics of varicose veins: Possible role of metalloproteinases. J. Vasc. Surg. 2003, 38, 162–169.
- Raffetto, J.D.; Qiao, X.; Koledova, V.V.; Khalil, R.A. Prolonged increases in vein wall tension increase matrix metalloproteinases and decrease constriction in rat vena cava: Potential implications in varicose veins. J. Vasc. Surg. 2008, 48, 447–456.
- Herouy, Y.; Mellios, P.; Bandemir, E.; Dichmann, S.; Nockowski, P.; Schöpf, E.; Norgauer, J. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J. Dermatol. Sci. 2001, 25, 198–205.
- Herouy, Y.; May, A.E.; Pornschlegel, G.; Stetter, C.; Grenz, H.; Preissner, K.T.; Schöpf, E.; Norgauer, J.; Vanscheidt, W. Lipodermatosclerosis is characterized by elevated expression and activation of matrix metalloproteinases: Implications for venous ulcer formation. J. Investig. Dermatol. 1998, 111, 822–827.
- Vaalamo, M.; Leivo, T.; Saarialho-Kere, U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum. Pathol. 1999, 30, 795–802.
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359.
- Rao, B.G. Recent developments in the design of specific Matrix Metalloproteinase inhibitors aided by structural and computational studies. Curr. Pharm. Des. 2005, 11, 295–322.
- Vihinen, P.; Ala-aho, R.; Kahari, V.M. Matrix metalloproteinases as therapeutic targets in cancer. Curr. Cancer Drug Targets 2005, 5, 203–220.
- Acharya, M.R.; Venitz, J.; Figg, W.D.; Sparreboom, A. Chemically modified tetracyclines as inhibitors of matrix metalloproteinases. Drug Resist. Updates 2004, 7, 195–208.
- Zakeri, B.; Wright, G.D. Chemical biology of tetracycline antibiotics. Biochem. Cell Biol. 2008, 86, 124–136.
- Morales, R.; Perrier, S.; Florent, J.M.; Beltra, J.; Dufour, S.; De Mendez, I.; Manceau, P.; Tertre, A.; Moreau, F.; Compere, D.; et al. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J. Mol. Biol. 2004, 341, 1063–1076.
- Steinmann-Niggli, K.; Ziswiler, R.; Kung, M.; Marti, H.P. Inhibition of matrix metalloproteinase attenuates anti-Thy1.1 nephritis. J. Am. Soc. Nephrol. 1998, 9, 397–407.
- Ermolli, M.; Schumacher, M.; Lods, N.; Hammoud, M.; Marti, H.P. Differential expression of MMP-2/MMP-9 and potential benefit of an MMP inhibitor in experimental acute kidney allograft rejection. Transpl. Immunol. 2003, 11, 137–145.
- Aggarwal, H.K.; Jain, D.; Talapatra, P.; Yadav, R.J.; Gupta, T.; Kathuria, K.L. Evaluation of role of doxycycline (a matrix metalloproteinase inhibitor) on renal functions in patients of diabetic nephropathy. Ren. Fail. 2010, 32, 941–946.
- Mosorin, M.; Juvonen, J.; Biancari, F.; Satta, J.; Surcel, H.M.; Leinonen, M.; Saikku, P.; Juvonen, T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: A randomized, double- blind, placebo-controlled pilot study. J. Vasc. Surg. 2001, 34, 606–610.
- Lindeman, J.H.; Abdul-Hussien, H.; van Bockel, J.H.; Wolterbeek, R.; Kleemann, R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: Doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 2009, 119, 2209–2216.
More
