Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Mona Zou and Version 1 by Christian Muehlenfeld.
Additive manufacturing (AM), popularly known as three-dimensional printing (3DP), has been widely applied to the fabrication of prototypes through to functional parts in various manufacturing areas, including the pharmaceutical industry. The term “3D printing” technically refers to a broad collection of additive technologies based on the deposition of materials, like creating objects from the bottom up or layer by layer. Despite the technical differentiation between the terms, “3D printing” has become the common term.
- 3D printing
- critical material properties
- polymers
- cellulosics
- vinylpyrrolidone
- bioresorbable polymers
- printability
- feedability
- drug product
1. Cellulosic Polymers
Cellulose derivatives are important excipients widely used in the pharmaceutical industry. This section provides an overview of pharmaceutically relevant cellulose ethers and their applications in AM. Cellulose is a polysaccharide of natural origin, composed of linear chains of 1-4-linked β-d-anhydroglucopyranose units of variable length, generally synthesized by plants, with wood and cotton fibers being the primary sources of cellulose for pharmaceutical applications. Due to intramolecular and intermolecular hydrogen bonds, cellulose is not soluble in water, although it is a highly hydrophilic polymer due its high degree of crystallinity and structure [115][1].
The etherification of the hydroxyl groups in the glucose ring is a strategy that changes the cellulose’s physicochemical mechanical properties (Figure 21). Carboxymethyl cellulose sodium (CMC), hydroxyethyl cellulose (HEC), hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HPC), and ethyl cellulose (EC) are the most used cellulose derivatives in pharmaceutical formulations [116,117][2][3]. The main differences between the different cellulose ethers depend on the chemistry, degrees of substitution, and distributions of the substituted groups as well as the molecular weight [118][4]. These properties influence solubility, viscosity in solution, surface activity, gelling performance, rheological behaviors in melt and solution, and mechanical characteristics, and thus make different cellulose ethers suitable for different 3D-printing applications.
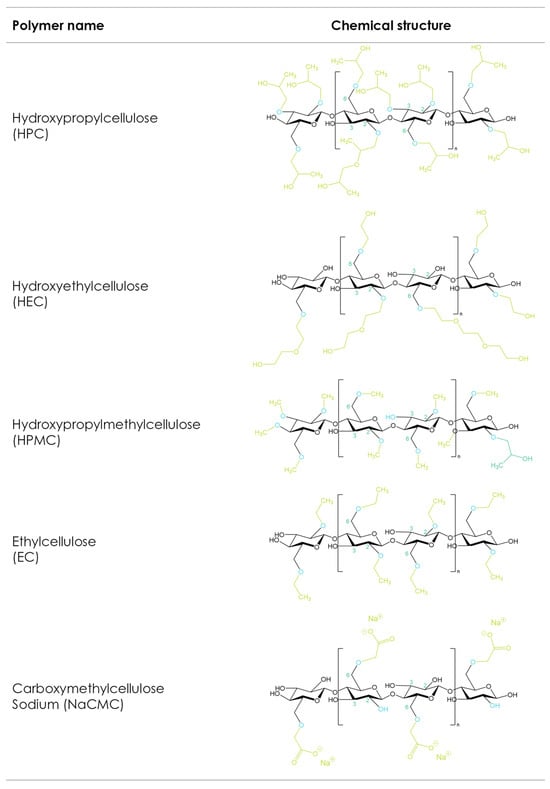
Figure 21. Cellulose ether derivatives.
1.1. Carboxymethyl Cellulose Sodium (CMC)
Carboxymethyl cellulose sodium (CMC) is the sodium salt of the carboxymethyl ether of cellulose, formed by the reaction of cellulose with monochloroacetic acid. During the synthesis of CMC, the hydroxyl groups of the anhydroglucose unit are substituted by carboxymethyl groups. Modification of the molecular weight (MW) of CMC leads to a multitude of grades with variable viscosities, with the average MW ranging from approximately 49,000 to 725,000 Da [22][5]. Furthermore, CMC grades can be classified based on their degree of substitution (DS), which is a crucial factor in determining the physical properties of CMC, having a direct impact on material properties such as solubility, rheology, and salt tolerance. As can be seen from the structural formula in Figure 21, there are three hydroxyl groups in each anhydroglucose unit in cellulose. The number of hydroxyl groups substituted per anhydroglucose unit in any reaction is defined as the degree of substitution or DS. In the case of CMC, the maximum theoretical DS would be 3.0 if all three hydroxyls were replaced by carboxymethyl; however, this is impossible in practice. The pharmaceutically relevant grades of CMC range from DS values of 0.7 and 0.9 to 1.2. While the corresponding sodium content for DS 0.7 and 0.9 ranges from 6.5 to 9.5% (w/w), the higher DS value of 1.2 results in a higher sodium content of 10.4–12% (w/w).
In recent years, AM has been used to develop 3D-printed biocompatible structures for drug-delivery and tissue-engineering applications; however, this requires the development of new biocompatible (hydrogel) inks. CMC is a promising candidate for the preparation of hydrogels (inks) since it is a natural, biocompatible, and biodegradable polymer and has good solubility in water with multiple carboxyl groups. Among all cellulose ethers, CMC, in particular, has recently been reported as a useful structural component of bioinks for wound healing due to its matrix-forming capability, cell compatibility, and crosslinking feasibility [119][6]. Despite all these benefits, CMC-based inks might show insufficient mechanical stability for printed structures and, thus, the incorporation of additional additives might be necessary [120][7]. Diaz-Gomez et al. developed 3D-printed scaffolds for the healing of diabetic wounds utilizing CMC. Different concentrated CMC dispersions ranging from 10 to 20% (w/v) were assessed for their printability. Among those, the 15% (w/v) CMC–citric acid ink showed sufficient rheological properties and stability during storage, thus bringing the project to the next phase, which includes clinical trials [29][8]. Wang et al. modified CMC with glycidyl methacrylate (GMA) and ε-polylysine (CP), resulting in hydrogels with a high compression modulus (238 kPa), stable rheological properties, and effective degradability [121][9]. Cui et al. selected CMC to form a fine paste with good flowability, extrudability, and buildability to prepare rapid-release tablets with a smooth appearance and sufficient mechanical properties [28][10]. Panraksa et al. utilized a syringe extrusion 3D-printing technique to study the feasibility of 3D-printed orodispersible films (ODFs). The authors evaluated different hydrophilic film-forming polymers, including HPMC, HEC, and CMC, as printing materials. Among the formulations evaluated, 5% (w/v) CMC solutions showed good printing resolutions and accurate dimensions. Furthermore, the 3D-printed ODFs containing CMC showed disintegration within 3 s, probably related to the high wettability, roughness, and porosity on the surface of the ODF [30][11]. All these applications demonstrated that CMC-based hydrogels are successful facilitators in 3D printing for tissue engineering and drug-delivery systems. On the contrary, the use of CMC is limited in material jetting and FDM-based AM technologies because of the poor thermal plasticity of the polymer.
1.2. Hydroxypropyl Methylcellulose (HPMC)
Hydroxypropyl methylcellulose or hypromellose (HPMC) is a cellulose ether prepared by reacting alkali cellulose in two steps: first, methyl chloride is added to introduce methoxy groups, followed by propylene oxide to introduce hydroxypropyl groups. Since the added hydroxypropyl group introduces a secondary hydroxyl group that can also be etherified during the manufacturing of HPMC, several types of HPMC are available with varying degrees of substitution (DS) and molar substitution (MS). The European pharmacopeia, USP/NF, and other pharmaceutical compendia differentiate the different substitution types of HPMC using four-digit numbers behind the non-commercial name (Figure 32), with the first two digits referring to the approximate percentage content (per weight) of the methoxy groups and the second two digits referring to the approximate percentage content of the hydroxypropoxy groups. From a commercial point of view, these different grades of HPMC were simplified by using single letters instead of four-digit numbers, i.e., “K” for HPMC 2208, “E” for HPMC 2910, and “F” for HPMC 2906. The molecular weight for all types of HPMC is in the range of 10–1500 kDa [117][3]. Since the viscosity of a HPMC solution corresponds to its molecular weight, the commercially available types add a suffix indicating the viscosity of a 2% aqueous solution (w/w) at 20 °C, followed by a designated “M” (multiplier of 1000) if needed, i.e., Benecel™ K100M hypromellose.
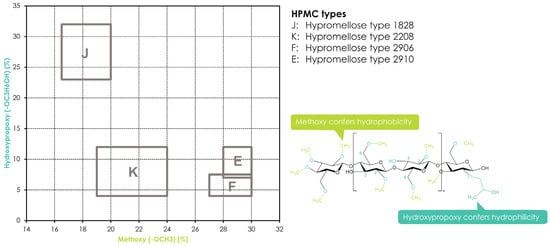
Figure 32. Substitution of HPMC and its different substitution types according to USP/NF and EP pharmacopeias. The rectangles in the graph depict the compendial ranges for MeO and HPO for the different HPMC types.
Variations in the fundamental structural properties of HPMC such as molecular weight, degree of substitution (DS), and the substitution pattern, which are due to the chemical structure of the polymer, will impact material properties such as elastic modulus, plasticity, glass transition temperature (Tg), and thermal gelation temperature in aqueous solutions, but they will also affect swelling, diffusion, and drug-release rate in pharmaceutical dosage forms [22,122][5][12]. HPMC has been widely used to form swellable–soluble matrices in hydrophilic oral sustained-release formulations since it is a water-soluble, non-ionic cellulose derivative and its solutions are stable within the pH range of 3–11.
Furthermore, HPMC-based matrices are non-cleavable by enzymes and thus are not affected by the pH of the gastrointestinal fluids, leading to robust and reproducible drug-release profiles in vivo. Recently, HPMC has gained interest in new applications such as AM due to its thermal gelation properties: while HPMC is soluble in cold water, forming a viscous colloidal solution, it becomes less soluble in water and will build up a gel upon heating. This thermo-reversible sol–gel transition is due to hydrophobic interactions between the methoxyl groups of hypromellose during polymer dehydration at high temperatures [123,124][13][14]. Accordingly, this behavior is not only relevant for HPMC but also for other cellulose ethers containing methoxyl groups, such as hydroxypropyl cellulose. The temperature at which this effect happens is called the gelation point and is a function of the HPMC type (number of methoxy groups) and concentration (ionic strength of the solution) ranging from 50 to 90 °C. While HPMC E types (2910) have a gelation point in the range of 65 °C, necessitating higher water temperatures for solution preparation [125][15], HPMC types with lower numbers of methoxyl groups will show lower thermogelation, respectively. The gelation of HPMC solutions with increasing temperature comes along with a visible change from a homogeneous solution to separated aqueous and polymer phases. This effect is called the cloud point and visualizes the phase transition due to the association of the polymer with relatively large aggregates [122][12]. It is noteworthy to mention that the cloud point and the gelation temperature of hypromellose are not necessarily the same, and the cloudiness of the solution may be observed prior to the gelation point in many cases.
However, since the gelation process is strictly reversible and HPMC will redissolve upon cooling, this thermally induced sol–gel transition makes HPMC a suitable ingredient for 3D-printing inks, as shown by Cheng et al., who studied the impact of the rheological properties of HPMC-containing hydrogels. Using theophylline as a model drug, the authors investigated the impact of the polymer on the printability and printing quality of their fabricated tablets. Two grades of HPMCs (E4M and K4M) were evaluated at different concentrations. A concentration of 12% (w/w) HPMC K4M in the hydrogel was recognized as the best carrier to print flexible dosage combinations with theophylline due to its great extrudability and shape-retention ability. The 3D-printed HPMC tablets showed sustained drug release over 12 h via both diffusion and erosion mechanisms from the matrix [74][16]. Elbadawi et al. used pressure-assisted micro-syringe (PAM) 3D printing to print films containing pullulan (PUL), using HPMC as a delivery system. HPMC was found to improve the mechanical properties of the PUL films, increasing tensile strength from 8.9 to 14.5 MPa and elastic moduli up to 1.56 GPa, respectively [25][17].
Different from CMC and HEC, HPMC exhibits some degree of thermal plasticity when the processing temperature is above its Tg, which is around 170–198 °C. The high Tg values, along with a relatively high melt viscosity, make it difficult to use HPMC without plasticizers [116][2]. While most available HPMC grades are unsuitable for thermal extrusion processes alone, certain grades and combinations with other thermoplastic polymers and/or plasticizers help to facilitate the extrusion process to obtain filaments with adequate mechanical properties for 3D printing. Zhang et al. combined hot-melt extrusion (HME) and FDM 3D printing using HPMC E5 and K100M together with acetaminophen (APAP) as a model drug. Filaments were manufactured by hot-melt extrusion and then transferred to the FDM printer. Solid-state characterization showed that the API was dissolved or molecularly dispersed into the polymer matrix and thus formed a solid dispersion. While the HPMC E5 was found to be a suitable filament for 3D printing with good flexibility, toughness, and stiffness, the higher-molecular-weight HPMC K100M could not be printed due to its high molecular weight and thus high melt viscosity. Both grades of HPMC required higher HME processing temperatures compared to other cellulose derivatives. Furthermore, the fabricated tablets did not result in optimal processability, indicated by rough printing paths due to the relatively high viscosity of the HPMC grades tested [52][18]. While the results from Zhang et al. showed that the HPMC filaments displayed high stiffness (high breaking stress) and toughness (high breaking distance), the filaments had rough surfaces. Thus, it was possible to feed the HPMC filaments into the printer, but the printing process was difficult because of the filaments’ rough surfaces and high melt viscosity during FDM printing. In order to overcome the limitations of HPMC-based filaments with respect to printability, filaments were prepared using binary polymer blends of HPMC E5 with hydroxypropyl cellulose (HPC EF or LF grades) or ethyl cellulose (EC N14 grade) and were found to perform better than single-polymer-based formulations [53][19]. In another study, Kadry et al. [69][20] investigated the effect of geometry on the drug-release profiles of FDM-printed HPMC tablets. However, it is important to emphasize that the HPMC used in this study (Affinisol™ HPMC HME 15LV) was of a non-compendial HPMC grade, with a different polymer substitution architecture compared to the allowed ranges of USP and Ph. Eur. pharmacopeias. While it showed a similar degree of methoxy substitution and a similar viscosity to the comparable regular HPMC viscosity and substitution grade, there was an increase in total substitution. The total substitution of the HPMC HME polymer grades was 47.0–59.0% yielding a Tg of around 90 °C [126][21]. This is comparable to the case of ethyl cellulose—a polymer with a typical total substitution of 48–49.5% and a Tg of around 133 °C. However, by successfully varying the geometric parameters, patterns, and infill densities of a drug-containing core, Kadry et al. [69][20] achieved different release profiles from the tablets.
1.3. Hydroxypropyl Cellulose (HPC)
Hydroxypropyl cellulose (HPC) is cellulose ether manufactured by reacting alkali cellulose with propylene oxide at an elevated temperature and pressure. It is a highly substituted cellulose ether since the added hydroxypropyl group containing an active hydroxyl group can be further etherified during the manufacturing process, resulting in additional side-chain extension. As a result, the molar substitution (MS), which refers to the number of moles of hydroxypropyl groups per anhydroglucose ring, will be higher than the degree of substitution (DS) [22][5]. The MS of HPC ranges typically between 3.4 and 4.1, and thus, the hydroxypropyl substituents comprise up to 80% of the weight of HPC. The high MS results in significant changes in material properties compared to other water-soluble cellulose ethers, e.g., HPC is significantly more thermoplastic and less hygroscopic than HPMC. Furthermore, it is fully soluble in water and polar organic solvents (ethanol, methanol, isopropyl alcohol, acetone), combining several hydrophobic and hydrophilic material properties. Because of these properties, HPC is a suitable candidate for solution-based printing inks, as used in PAM printing. Sjöholm et al. fabricated thin orodispersible HPC films with a printing ink comprised of 16% (w/w) low-molecular-weight HPC EF in a solution of water and ethanol [66][22]. Cui et al. [67][23] prepared high-drug-loaded tablets (96% w/w levetiracetam) in different geometrical shapes by adding 2% of a medium-molecular-weight-grade HPC MF as a binder and 2% croscarmellose sodium as a disintegrant. The 3D-printed tablets showed acceptable ranges for tablet-breaking force, tablet friability, weight variation, and drug content. Abdella et al. fabricated estradiol-containing films using a formulation containing HPC in PAM printing. The results indicated that different infill patterns affected the film’s mechanical properties and its drug-release kinetics [75][24].
Commercially available grades of HPC are available in different MW grades, with values ranging from 20,000 to 1,500,000 Da (Table 31).
Table 31. Example of the commercially available grades of hydroxypropyl cellulose (HPC) with data from the manufacturer, Ashland.
| Viscosity Grade | Weight Average Molecular Weight (Da) | Viscosity (mPa∙s) in Aqueous Solution | Solution Concentration (%) | Grade Used in 3DP Applications (Based on MW) |
|---|---|---|---|---|
| HF | 1,150,000 | 1500–3000 | 1 | [52][18] |
| MF | 850,000 | 4000–6500 | 2 | [46,55,67][23][25][26] |
| GF | 370,000 | 150–400 | 2 | |
| JF | 140,000 | 150–400 | 5 | [62][27] |
| LF | 95,000 | 75–150 | 5 | [39,53,64][19][28][29] |
| EF | 80,000 | 300–600 | 10 | [40,52,53,63,66][18][19][22][30][31] |
| ELF | 40,000 | 150–300 | 10 | [24,43,][27][57,32][62,33][34]65[35] |
Within a specific viscosity grade, HPC is further available in different particle size grades. Infanger et al. [24][32] evaluated four different HPC qualities of two different viscosities on a drop-on-powder (DoP) printer. While the dissolution and disintegration behavior of the 3D-printed tablets mainly depended on the MW (and thus, viscosity) of the HPC grade used, the friability mainly depended on the particle size of the employed binder. Finer particle size grades resulted in less-friable tablets, and higher-MW HPC grades resulted in slower dissolution and disintegration times due to their higher viscosity and slower hydration rate. Goyanes et al. fabricated solid dispersions of itraconazole as a model drug using different grades of HPC, with MW ranging from 20 to 140 kDa. As the results of Infanger suggested, the fabricated tablets comprised of the lower-MW HPC exhibited faster drug release compared to the grades with higher MWs [62][27].
In addition to HPC’s good solubility in different solvents, it also shows excellent thermal plasticity and thermostability (with a degradation temperature of 227 °C [127][36]) due to its unique molecular structure, making it suitable for processes that require melting and extrusion, such as fused deposition modeling (FDM). In contrast to other cellulosic polymers, there is only inconsistent information on the Tg of HPC available, with various Tg values reported. Rials and Glasser [128][37] observed that HPC is a semicrystalline material rather than a fully amorphous one, showing a Tg of 19 °C and a melting point of 220 °C. Picker-Freyer and Durig [129][38] also concluded that HPC is a semicrystalline material, where they observed Tg values in the range of 0 to 5 °C in the presence of 1–10% moisture in the polymer. The marked reduction in the Tg as moisture increases was connected to increased plasticity with an increasing moisture content. Meena et al. [127][36] reported a shallow baseline shift in the DSC scan of the polymer at 111 °C, which they attributed to Tg. Luebbert et al. [130][39] determined the Tg of different low-molecular-weight (MW) HPC grades via extrapolating the glass transition of spray-dried HPC/copovidone blends. The Tg varied from 81.6 to 84.2 °C with increasing MW. The complex morphological structure of HPC might account for the difficulties in determining the Tg of HPC. Anyhow, the low Tg of HPC results in low melt viscosity and fast melt-flow properties (depending on the MW of the polymer); thus, HPC enables formulators to prepare filaments and print them at a relatively low temperature without the help of a plasticizer. Low-molecular-weight grades are processable at temperatures as low as 120 °C, while high-molecular-weight grades are processable at 200 °C without the use of a plasticizer. In addition, extruding at different temperatures and molecular-weight grades also affects the toughness and flexibility of HPC [131][40]. All these properties make HPC a suitable polymer for melt extrusion-based AM techniques.
1.4. Hydroxyethyl Cellulose (HEC)
Hydroxyethyl cellulose is a cellulose ether prepared from the reaction of alkali cellulose and ethylene oxide. Each added hydroxyethyl group introduces a reactive secondary hydroxyl group that can be further etherified during the manufacturing of HEC. Like HPC, HEC can be further substituted, resulting in an additional chain extension. HEC has found widespread use in pharmaceuticals as a hydrophilic matrix-former, binder, thickener, and film-former, and its ease of solubility (both in hot and cold water as well as many organic solvents) makes it an appropriate candidate in many biomedical applications, e.g., different grades of HEC with varying MWs and viscosities have been successfully used to adjust the viscosity of 3D-printing inks. Gospodinova et al. used HEC-based bioinks in an extrusion-based 3D bioprinting process. HEC-containing hydrogels were prepared at a concentration of 5% (w/v), and sodium alginate (SA) was added in concentrations of 1%, 2.5%, and 5% (w/v), respectively. Extrudability and shape fidelity served as parameters to assess the printability of the hydrogels. Extrudability in this context refers to the lowest pressure at which the hydrogel could be extruded with a reasonable flow rate for the printing process [59][41].
Elbl et al. [60][42] used different grades of HEC as thickening agents for a modified FDM technique, in which the FDM extruder was replaced by a linear syringe pump. Low viscosity and surface tension of the printing dispersion can cause insufficient uniformity of the drug content and unsatisfactory mechanical properties of films due to uneven distribution of the ingredients in the ink paste. The different MW grades of HEC were used with a concentration of 1% (w/w), leading to printable films with a smooth surface [60][42]. Luo et al. used 3D-printing technology to fabricate bilayer films comprised of chitosan (CS) and HEC. The best-performing ink was identified to have a CS/HEC ratio of 3:3 and a suitable apparent viscosity, resulting in a consistent printing process with no breakage or clogging observed [61][43].
Compared to HPC and EC, and even HPMC, the poor thermoplastic behavior of HEC might explain the lack of available studies for HEC in thermal manufacturing processes, such as FDM-based 3D printing. Hartzke et al. [57][34] evaluated the processability of different grades of HECs in FDM printing and figured out that extrusion of HEC alone was not possible. To overcome this process limitation, the authors mixed HEC (75% w/w) with thermoplastic HPC (20% w/w) to improve the overall thermoplastic behavior of the mixture. Together with diclofenac (5% w/w) as a model API, the ternary mixture enabled the successful FDM printing of tablets. This demonstrates the importance of blending polymers with different properties to facilitate processability based on the requirements of the AM process. The authors concluded that low-viscosity HEC of grade 250 L with a low MW was the most suitable, resulting in sufficient feedability and printability of the filament and the high breaking force of the fabricated tablets [57][34]. Fina et al. [58][44] included high-MW-grade HEC 250H as a matrix-forming suspending agent in a formulation comprised of HPC, polyethylenoxide (PEO), and mannitol. During dissolution of the tablet, the dissolution medium dissolves both mannitol and hydroxypropyl cellulose, resulting in void spaces inside. This allows PEO to swell and, thus, create a microenvironment where the drug is suspended and diffuses out slowly. HEC was included at a level of 5% w/w in the core composition to prevent drug sedimentation [58][44].
1.5. Ethyl Cellulose (EC)
Ethyl cellulose (EC) is a partly O-ethylated cellulose ether derivative that is manufactured by the reaction of alkali cellulose with ethyl chloride at approximately 60 °C for several hours [132][45]. The substitution level or the ethoxyl content directly impacts the properties of the resulting EC. A typical structure of EC has a DS value of 2.5 per anhydroglucose unit, corresponding to a 48.5 wt.% ethoxyl content. Currently, the major pharmacopeias (USP/NF and EP) define the ethoxyl content of EC to be between 44.0 and 51.0% (w/w), and various ethyl cellulose types are distinguished by their molecular weight or viscosity, respectively (Table 42). Like other cellulose ethers, the physical properties of EC depend on the MW, its degree of etherification, and the distribution pattern of the substituted groups.
Table 42. Available pharmaceutical grades of ethyl cellulose according to Brady et al. [22][5] and 3DP publication references.
| Grade | Ethoxyl Substitution % | Average Molecular Weight | Viscosity | Solution Concentration (%) | Grade Used in 3DP Applications |
|---|---|---|---|---|---|
| N7 | 48.0–49.5 | 65,000 | 6–8 | 5 | [50,51][46][47] |
| N10 | 48.0–49.5 | 75,000 | 8–11 | 5 | [38,39,55,56][26][28][48][49] |
| N14 | 48.0–49.5 | 120,000 | 12–16 | 5 | [52,[1853]][19] |
| N22 | 48.0–49.5 | 140,000 | 18–24 | 5 | |
| N50 | 48.0–49.5 | 160,000 | 40–52 | 5 | |
| N100 | 48.0–49.5 | 215,000 | 80–105 | 5 | |
| T10 | 49.6–51.0 | 75,000 | 8–11 | 5 |
EC is considered to be a biodegradable material, and in contrast to most other cellulose ethers, it is not soluble in water but can be dissolved in most organic solvents, and thus, EC-containing printing inks are solvent-based. Adams et al. dissolved different amounts of EC in an alpha-terpineol solvent to vary the viscosity of the resulting printing inks. The authors customized a direct ink writing printer for printing the EC solutions and demonstrated a steady flow for a range of EC inks with different viscosities, suitable for 3D printing. This process was utilized to successfully fabricate biopolymer parts [54][50].
Kavimughil et al. formulated an 11% (w/w) EC-based oleogel using medium-chain triglyceride and stated acceptable printability via an oil-binding capacity test. The authors assessed printability at different temperatures, and 45 °C was reported to provide the best printability and process performance. The study highlighted the potential of oleogel systems for AM applications, with the improved bioaccessibility and bioavailability of hydrophobic/lipophilic active ingredients [133][51].
Yu et al. [47,48,49][52][53][54] used ethyl cellulose among other polymers in DoP binder jetting applications. In one of the cases [49][54], EC was used to print multi-layered doughnut-shaped drug-delivery devices, where the API was sandwiched between ethyl cellulose-rich, drug-free top and bottom barriers, enabling a linear drug-release profile. EC served as the retarding polymer controlling the drug dissolution rate; however, it also helped to provide strong adherence forces with the drug-loaded regions in the printed shape and eliminate the burst effect in dissolution testing.
EC exhibits a relatively high Tg at 132 °C, has a melting point Tm of around 180 °C (depending on the polymer MW), and its storage modulus remains in a glassy state up to 64 °C, indicating rigid filaments at room temperature. Therefore, EC is a suitable polymer for FDM filament printing that exhibits sufficient thermal plasticity and, thus, extrudability. In a study performed by Zhang et al. [53][19], modified-release tablets were prepared by FDM coupled with HME to fabricate filaments. The results indicated that the EC filaments were too brittle, and thus, they were broken by the FDM printer’s feeding gears during feeding. To improve the brittleness of the EC filaments, EC was mixed with other polymers, such as HPC, HPMC, copovidone (PVPVA), poly(vinyl alcohol), polyethylene glycol, or xanthan gum. Mixing EC with those polymers helped to adjust the EC filaments’ stiffness and brittleness and led to well-extrudable mixtures and good printable filaments [53][19]. Borujeni et al. mixed EC and HPC and evaluated the effect of different blends on the mechanical properties of extruded filaments and their printability. The best properties for FDM printing were achieved by a filament formulation containing CBZ, EC, and HPC (3%, 64.7%, and 32.3% w/w, respectively). This filament resulted in 3D-printed tablets with appropriate mechanical properties and good content uniformity in the API [55][26]. Shi et al. fabricated ibuprofen and EC-based matrix systems, combining various polymers and extruding them into filaments by twin-screw extrusion. The filaments were successfully printed using an FDM printer, and the mechanical characterization indicated that the filaments’ stiffness and brittleness were significantly improved by adding other polymers to the EC/ibuprofen matrix. Furthermore, in vitro dissolution studies showed that it was possible to control the drug release over 24 h by varying the additional polymer and its hydrophilicity [38][48]. Yang et al. [56][49] evaluated EC-based formulations to sustain the release of ibuprofen. Ratios of 50–80% (w/w) EC and 16–24% ibuprofen were blended with additional release modifiers such as HPMC, sodium alginate, xanthan gum, or polyvinyl alcohol before hot-melt extrusion at a 100–120 °C processing temperature. The FDM printability was greatly affected by the melt rheology and mechanical property of the filaments fed into the FDM printer; however, the targeted drug release within 24 h was achieved by an optimized formulation and printing process parameters affecting the infill pattern and density, as well as the shell thickness of the printed shapes. In conclusion, EC is a suitable material for HME/FDM 3D printing. Generally, lower-viscosity EC grades tend to show better thermoplastic properties than higher-MW grades, probably due to better alignment and less steric hindrance in low-MW grades [27,117][3][55].
2. Polyvinyl Polymers
Polyvinyl polymers are synthetic amorphous linear polymers synthesized from formaldehyde and acetylene using the Reppe process to obtain N-vinylpyrrolidone (NVP) over different synthesis steps [134][56]. NVP is consequently polymerized either alone (to obtain homopolymers) or with other monomers (to obtain copolymers) using a free-radical polymerization step, induced by the addition of an initiator, which controls the final molecular weight of the synthesized polymer. Polyvinylpyrrolidone (PVP) is obtained from the polymerization of NVP solely, while vinyl acetate-vinylpyrrolidone copolymer (PVPVA) is obtained from the polymerization of NVP with vinyl acetate. Figure 43 shows exemplary chemical formulas for both polymers. Both polymers show favorable physicochemical characteristics, such as solubility in a wide variety of solvents, including water; ability to interact both with lipophilic and hydrophilic substances [135][57]; good adhesion properties; thermoplasticity; and low toxicity [136][58]. Consequently, they have been broadly used in pharmaceuticals—for example, as tablet binders [137][59], thickeners, and in the solubilization of drugs by forming amorphous solid dispersions [138][60]. In pharmaceutical AM, PVP and PVPVA have been used as suitable excipients in FDM, SLS, and binder jetting technologies.
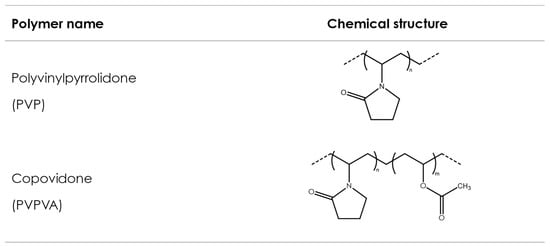
Figure 43. Polyvinylpyrrolidone and copovidone.
2.1. Polyvinylpyrrolidone (PVP)
Polyvinylpyrrolidone (PVP) is also known by its compendial name povidone. It is available in different molecular-weight grades, reflecting the number of repeating vinylpyrrolidone units. Historically, difficulties in the determination of the molecular weight of this polymer led to the introduction of the K-value concept, as a reference to the molecular weight. The K-value, also known as the Fikentscher viscosity coefficient, is calculated from the kinematic viscosity of an aqueous polymer solution, measured using a capillary viscosimeter [139][61], and its concentration:
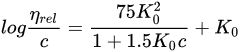
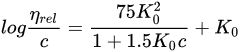
In this equation, the relative viscosity of the aqueous solution compared to that of the solvent is represented by ηrel, the concentration of the solution in g/100 mL is given by c, and K0 is the K-value multiplied by 10−3. The Fikentscher equation can also be rearranged, and thus, the K-value can be directly calculated, as shown in Equation (2):

Different pharmacopoeias, such as the USP-NF, EP or JP, accept that the K-value calculated by Fikentscher’s equation indirectly represents the molecular weight (MW) of the polymer and the viscosity of its solutions. Still, other methods such as gel permeation chromatography, light scattering, and vapor pressure osmometry have been used for determining the MW, and thus, the resulting representation of MW would be different depending on the used method of determination (weight average, MW; intrinsic viscosity; or number average, Mn) [134,140][56][62]. Table 53 shows different grades of PVP along with their typical K-value ranges and molecular weights, determined with different methods.
Table 53. Different grades of PVP with their K-values, molecular weights and glass transition temperatures (data from [134,141][56][63]).
| Grade | Nominal K-Value | K-Value Range a | Calculated Relative Viscosity of 10% (w/w) Solution (mm²/s) b | Intrinsic Viscosity (dL g−1) | MW (Dalton) |
Mn (Dalton) |
Tg (°C) |
|---|---|---|---|---|---|---|---|
| PVP K-12 | 12 | 10.2–13.8 | 1.48–1.8 | 0.05 | 2500 e | 1300 | 120 |
| PVP K-17 | 17 | 15.3–18.4 | 1.98–2.41 | 0.09 | 10,000 c | 2500 d | 140 |
| PVP K-25 | 25 | 22.5–27 | 3.23–4.56 | 0.16 | 25,000 c | 6000 d | 160 |
| PVP K-30 | 30 | 27–32.4 | 4.56–7.14 | 0.22 | 40,000 c | 10,000 d | 164 |
| PVP K-90 | 90 | 81–97.2 | 1075.37–7157.85 | 1.6 | 1,100,000 c | 150,000 e | 174 |
a according to the European Pharmacopeia, b calculation based on Equation (1), c light scattering; d vapor pressure osmometry; e gel permeation chromatography.
The solution viscosity, glass transition temperature, and adhesive properties of the polymer typically increase with increasing molecular weight. On the contrary, the dissolution rate of the polymer increases when the molecular weight decreases, due to the viscosity reduction of their solutions. The melting point temperature (Tm), glass transition temperature (Tg), melt index, and thermal degradation temperature (Tdeg) are fundamental characteristics related to the thermoplastic behavior of polymers. Table 53 illustrates the relationship between the Tg and molecular weight or K-value, respectively. Similar to the Tg, melting temperatures and melt viscosity increase with increasing molecular weight [22][5]. These polymer properties are also relevant for some 3D-printing technologies, e.g., FDM printing. For melt-based process technologies, the polymers should possess thermoplastic properties to allow printing; however, they need to be processed in temperature ranges in which the polymers remain thermally stable. Therefore, polymers with a wide thermal processing window (typically the temperature range between the polymer’s Tg or extrudability temperature and its degradation temperature) should be selected for optimized filament feeding. Still, the use of PVP in FDM remains challenging due to its limited extrudability in the absence of an appropriate plasticizer or other ingredients with a plasticizing effect on the polymer. The resulting filaments are often brittle, causing difficulties in the feedability of the filament into the printer.
Numerous studies describe the use of PVP in 3DP. Kollamaran et al. [41][64] combined a PVP (low-molecular-weight grade) with copovidone in formulations containing ramipril, with the aim of achieving an immediate-release (IR) profile of the printed drug product. The addition of the low-MW PVP lowered the necessary printing temperature during the FDM process, allowing for the printing of a thermolabile and low-melting drug like ramipril without degradation. Moreover, the formulations combining low-MW PVP with copovidone showed a slightly faster drug release than those containing just copovidone.
As mentioned before, as the molecular weight of the polymer increases, the solution viscosity increases (Table 53). PVP is water soluble, its maximal solubility being limited only by the viscosity of its own solution. Compared to most cellulose ethers, solutions of low-MW-grade PVP have an exceptionally low viscosity. PVP is also nonionic and its viscosity in aqueous solutions is not affected by the pH or by salts. These properties allowed the preparation of an aqueous PVP-based ink for solvent inkjet printing (DoD) [23][65].
Due to its properties, PVP is also an important excipient in binder jetting technologies. One of the first publications using PVP in this application combined it with small amounts of maltitol and maltodextrin to print fast-dissolving tablets of hydrophilic captopril [94][66]. The first ever approved 3D-printed drug, Spritam® (Aprecia Pharmaceuticals, Blue Ash, OH, USA), was also prepared using binder jetting and PVP [4][67]. While the tablet matrix contains 60–90% (w/w) of the drug levetiracetam along with microcrystalline cellulose, mannitol and colloidal silicon dioxide, the matrix materials are bound together by an aqueous solution of PVP, glycerin, and a surfactant. Tablets prepared in this manner contain twice as much API compared to conventionally manufactured tablets. The fabricated tablets are highly porous, resulting in a short disintegrating time, leading to a noticeable improvement in patient compliance. Studies comparing different binders in similar process conditions were conducted by Lee et al. [94][66] and Tian et al. [100][68]. The results of both studies indicated that 3D-printed tablets containing PVP had an appropriate tablet hardness; however, their disintegration time was longer than expected. Another study using a DoP printing process conducted by Sen et al. [96][69] focused on the mechanical strength of printed tablets containing amitriptyline HCL as a model drug. Combinations of eight fillers and ten binders were studied. The formulation comprised of lactose monohydrate and PVP K30 increased the mechanical strength of the printed tablets, most likely due to the formation of solid bridges. The authors also observed that not all tested binders provided the necessary strength to the tablets, with PVP being one of the few binders that performed well. Similar findings were reported by Tian et al. [95][70], who prepared orally disintegrating tablets containing warfarin as an API. They observed that the combination of D-sucrose and the PVP K30 as the filler and binder resulted in printed tablets with sufficient mechanical properties and an appropriate disintegration time. Kozakiewicz-Latała et al. presented a formulation approach that allowed for the manufacture of fast-dissolving tablets with a small dose (3 mg) of the hydrophilic model API quinapril hydrochloride. Mixing the API with microcrystalline cellulose as a bulking agent and PVP K25 as a binding agent led to porous fast-dissolving tablets with satisfactory mechanical properties [97][71].
Povidone has been used in PAM printing processes as well, e.g., Dores et al. [106][72] compared tablet formulations containing 40% (w/w) theophylline as a drug; PVP or PVA as a polymer; and lactose, D-Mannitol, and sorbitol as highly soluble fillers/plasticizers. Tablets containing PVP and lactose tend to show a more cohesive structure compared to tablets made with PVA. In another study, Khaled et al. fabricated polypills consisting of different compartments to adjust the release profiles of three APIs [71][73]. PVP was used as a binder for the immediate-release compartment. Abdella et al. [75][24] demonstrated that PVP can be used to prevent the separation/sedimentation of the drug from the suspension used during the printing process. The addition of PVP increased the viscosity of the printed mucoadhesive buccal film and, thus, helped suspend the API in the film-forming solution.
2.2. Copovidone (PVPVA)
Copovidone (PVPVA) is a random, linear copolymer produced by the free radical polymerization of vinylpyrrolidone and vinyl acetate (VP–VA copolymer; Figure 43) with a 6:4 ratio of VP to VA. It is a freely flowing, spray-dried powder with a spherical, hollow-particle morphology and was designed to overcome some of the limitations associated with polyvinylpyrrolidone. PVP, for example, is a relatively stiff, brittle, and hygroscopic material, although it is frequently used as a tablet binder. The brittleness and stiffness are reflected in the relatively high Tg of approximately 164 °C for a PVP (PVP K30), compared to a lower Tg of approximately 108–111 °C for PVPVA, with a molecular weight close to the MW PVP K30. The decrease in the Tg of copovidone due to the addition of a comonomer to vinylpyrrolidone improves the copolymer plasticity and flexibility, and thus, PVPVA has a significantly lower Tg and is a lot more flexible and plastic compared to PVP (Table 64).
Table 64. Common grades of commercially available copovidone with data from [142,143,144,145][74][75][76][77].
| Product Name | Manufacturer | Molecular Weight (Mn) | Tg | Particle Size (x50) |
|---|---|---|---|---|
| Plasdone™ S630 | Ashland | 14.000–18.000 | 110.69 | <100 |
| Plasdone™ S630 ultra | Ashland | 20.000 | 108.72 | <100 |
| Kollidon™ VA64 | BASF | 15.000–20.000 | 109 | 71.1 |
| Kollidon™ VA64 fine | BASF | 15.000–20.000 | 109 | 16.2 |
While most material properties of polymers, such as the MW, DS, nature of the functional groups, etc., are imparted by the synthesis step of the manufacturing process, there are also properties imparted after the synthesis (due to drying or milling), such as particle size, density, and particle shape. These properties can impact material characteristics such as powder flow and powder handling and are important for the processability of the polymers. As an example, the original, regular grades of copovidone (Plasdone™ S630 and Kollidon™ VA64, respectively) were improved by introducing a finer particle-size grade (Kollidon™ VA64 fine) for better tablet binding, e.g., in dry granulation by roller compaction [143][75], or by adjustments in powder flowability and particle size distribution, resulting in better processability in hot-melt extrusion processes (Plasdone™ S630 ultra) [142][74].
Furthermore, copolymerizing vinylpyrrolidone with vinyl acetate also reduces the hygroscopicity of PVPVA compared to PVP [134][56], resulting in up to three times less water absorption compared to PVP [146][78].
The combination of good thermoplastic properties and low Tg make copovidone a suitable polymer for melt-based processes (e.g., hot-melt extrusion), and, therefore, copovidone has been chosen for FDM processes as well. However, the inherent high brittleness of PVPVA can pose challenges to some printing processes. Henry et al. [40][30] tried to print copovidone filaments in FDM printing; however, they failed at successful printing because of this. From hot-melt extrusion (HME) applications, it is known that the processability of brittle polymers such as PVPVA can be significantly improved by combining them with plasticizers or other polymers, and consequently, this was adopted for FDM printing as well. Kollamaram et al. added a plasticizer, PEG 1500, to a PVPVA-based formulation to lower its Tg, and thus, they improved the processing performance of the formulation [41][64]; Solanki et al. [42][79] mixed PVPVA and HPMC to improve the mechanical properties of the filament and enable a successful printing process. In addition to plasticizers or other polymers, APIs can help to enable the printing of copovidone-based filaments as well, due to their plasticizing effect on the formulation. Ayyoubi et al. demonstrated a successful fabrication of spherical 6 mm mini tablets combining copovidone with nifedipine. A drug loading of 50% nifedipine helped to produce filaments by hot-melt extrusion that were able to be fed into the FDM printer [39][28]. Boniatti et al. [9][80] explored the use of direct powder extrusion to reduce the dependence on the strict mechanical properties of HME filaments for FDM printing. Tablets contained PVPVA and 35% or 50% (w/w) API, respectively, and were evaluated with a special focus on the drug dissolution profiles, physical stability, and taste-masking effectiveness.
Copovidone has also been utilized in binder jetting technologies. Chang et al. [98][81] reported the remarkably better performance of copovidone compared to PVP with respect to the binding strength and disintegration behavior. Gottschalk et al. used a drop-on-powder printer to fabricate tablets containing poorly water-soluble ketoconazole embedded in a PVPVA-based amorphous solid dispersion. While the amorphous solid dispersion was prepared via hot-melt extrusion with drug loadings of 20% and 40%, the actual printing took place after milling the extrudates to powdered material again. This approach can serve as an alternative to FDM printing and help to overcome the limited mechanical properties of the copovidone-based feedstock [31][82].
Selective laser sintering (SLS) is another technology where copovidone has been used with success: Fina et al. printed tablets with fast disintegration behavior using copovidone and low-dose paracetamol as a model drug (5% (w/w)). The authors investigated the influence of several printing parameters on the drug-release characteristics of the printed formulations. A faster laser scanning speed resulted in tablets that were completely dispersed in less than 4 s in a small volume of water, like conventional manufactured orally disintegrating tablets (ODTs). The authors concluded that the less-energetic sintering process at a faster laser scanning speed caused the powder particles in the fabricated tablet to better separate from each other upon contact with liquids, resulting in the faster disintegration time of the printed tablets. Furthermore, the higher porosity of tablets fabricated at faster laser scanning speeds shortened the disintegration time, as the dissolution medium was able to penetrate the porous tablet structure through capillary action, also commonly referred to as “wicking” [33][83]. Barakh Ali et al. [36][84] investigated the effects of formulation and process variables on the quality of diclofenac sodium-loaded printlets using SLS. The formulations consisted of 55–59% (w/w) PVPVA, 30% (w/w) API, 8–12% (w/w), and 3% (w/w) Candurin® NXT Ruby Red. The latter material was included to enhance the energy absorption from the laser and thus aid the printability of the formulation. Surface temperature, laser scanning speed, and lactose concentration were studied for their effect on the printlet quality, with all of them showing significant impact. The authors concluded that it was possible to fabricate printlets with good mechanical integrity and fast disintegration and dissolution rates using SLS. Allahham et al. [37][85] used PVPVA and Mannitol to print drug–cyclodextrin complexes. At a 100 °C printing temperature, copovidone reaches a rubbery state, and polymer particles connect to each other, forming bridges and sintered areas following the passage of the laser. While PVPVA already enters a rubbery state due to its Tg of around 109 °C at the printing temperature, Mannitol has a melting point of approximately 168 °C, and thus, the Mannitol particles either dissolve in the rubbery PVPVA or are unmodified, trapped within the PVPVA matrix. This combination allows for the fabrication of printlets with a high porosity, resulting in fast disintegrating times. Awad et al. [11][86] used 92% w/w PVPVA due to its good printability and fast disintegration properties. The authors manufactured personalized printlets with Braille or Moon patterns on their surface, targeting blind or visually impaired individuals. Mohamed et al. utilized SLS printing to fabricate tablets containing clindamycin palmitate HCl along with PVPVA, microcrystalline cellulose, and lactose monohydrate. The laser scanning speed was concluded to be the most important process factor, directly impacting the porosity of the tablet, thus impacting the dissolution rate and tablet disintegration as well as the tablet breaking force and crystallinity of the printed tablet [34][87]. Davis et al. [35][88] used a single-step SLS process to print copovidone-based amorphous solid dispersions with Ritonavir as a model drug. Process parameters affecting the melting of the composition such as surface temperature and hatch spacing were identified to have a significant impact on the ability to fabricate a fully amorphous product.
3. Bioresorbable Polymers—Aliphatic Polyesters
Aliphatic polyesters are a class of bioresorbable polymers that have been widely studied and employed in recent years [147][89]. Biodegradability is achieved due to an aliphatic ester bond on the polymer backbone, which is hydrolyzed in aqueous environments. Moreover, their degradation by-products are eliminated from the body via natural metabolic pathways, making these materials attractive for biomedical applications.
Although various types of aliphatic polyesters exist, such as naturally derived polyhydroxyalkanoates (PHAs) or poly(alkenedicarboxylate)s, this article focuses on the most commonly used synthetic aliphatic polyesters, often described as poly-α-hydroxy acids or poly-α-hydroxyesters, whose chemical structures are shown in Figure 54. These polymers have been used in a medical context for more than 50 years and are present in a large variety of medical products commercially available on the market with various applications, like implants for prolonged drug delivery, long-acting injectables (LAI) for extended drug release, orthopedic fixation devices, wound dressings, and scaffolds for tissue engineering [148,149][90][91]. However, such products put little focus on customization and, in general, take a one-size-fits-all approach. AM processes, such as 3D printing, make customization possible by allowing the production of dosage forms or implants with complex geometries, which fit the patient’s individual anatomy in a quick, cost-effective manner without sacrificing precision [150][92].
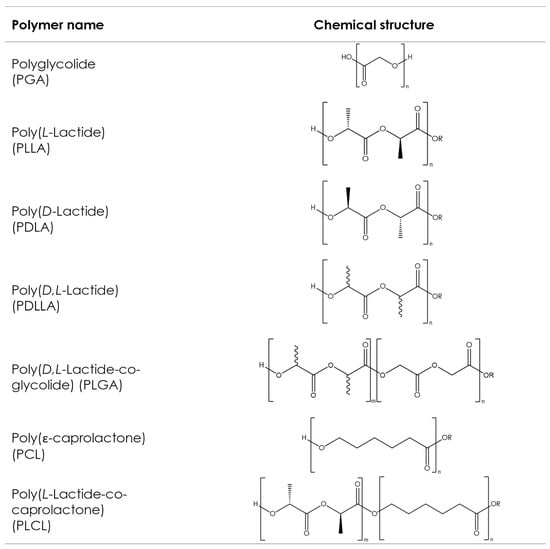
Figure 54. Chemical structures of the commonly used aliphatic polyesters. Bonds with wavy lines indicate that the stereochemistry of the bond is unknown.
The ring-opening polymerization (ROP) of cyclic di-ester monomers is the most commonly used method of synthesizing poly-α-hydroxyesters. While the polycondensation of respective difunctional acids can also be used [151[93][94],152], pharmaceutical applications typically require relatively high molecular weights, and this can only be easily achieved via ROP [153,154][95][96]. The physical, mechanical, and processing properties of these synthetic polyesters are mainly influenced by the type of monomers used and the final molecular weight [155][97], both of which are easily controlled during synthesis.
References
- Chavan, R.B.; Rathi, S.; Jyothi, V.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J Pharm Sci 2019, 14, 248–264.
- Funk, N.L.; Fantaus, S.; Beck, R.C.R. Immediate release 3D printed oral dosage forms: How different polymers have been explored to reach suitable drug release behaviour. Int. J. Pharm. 2022, 625, 122066.
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22.
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931.
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; Chapter 7; pp. 181–223.
- Mallakpour, S.; Tukhani, M.; Hussain, C.M. Recent advancements in 3D bioprinting technology of carboxymethyl cellulose-based hydrogels: Utilization in tissue engineering. Adv. Colloid Interface Sci. 2021, 292, 102415.
- Zennifer, A.; Senthilvelan, P.; Sethuraman, S.; Sundaramurthi, D. Key advances of carboxymethyl cellulose in tissue engineering & 3D bioprinting applications. Carbohydr. Polym. 2021, 256, 117561.
- Diaz-Gomez, L.; Gonzalez-Prada, I.; Millan, R.; Da Silva-Candal, A.; Bugallo-Casal, A.; Campos, F.; Concheiro, A.; Alvarez-Lorenzo, C. 3D printed carboxymethyl cellulose scaffolds for autologous growth factors delivery in wound healing. Carbohydr. Polym. 2022, 278, 118924.
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104.
- Cui, M.; Li, Y.; Wang, S.; Chai, Y.; Lou, J.; Chen, F.; Li, Q.; Pan, W.; Ding, P. Exploration and Preparation of a Dose-Flexible Regulation System for Levetiracetam Tablets via Novel Semi-Solid Extrusion Three-Dimensional Printing. J. Pharm. Sci. 2019, 108, 977–986.
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454.
- Sarkar, N. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. J. Appl. Polym. Sci. 1979, 24, 1073–1087.
- Haque, A.; Morris, E.R. Thermogelation of methylcellulose. Part I: Molecular structures and processes. Carbohydr. Polym. 1993, 22, 161–173.
- Haque, A.; Richardson, R.K.; Morris, E.R.; Gidley, M.J.; Caswell, D.C. Thermogelation of methylcellulose. Part II: Effect of hydroxypropyl substituents. Carbohydr. Polym. 1993, 22, 175–186.
- Dürig, T.; Karan, K. Binders in Pharmaceutical Granulation. In Handbook of Pharmaceutical Granulation Technology, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021.
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983.
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197.
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302.
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197.
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 2018, 544, 285–296.
- Khatri, P.; Katikaneni, P.; Desai, D.; Minko, T. Evaluation of Affinisol® HPMC polymers for direct compression process applications. J. Drug Deliv. Sci. Technol. 2018, 47, 461–467.
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123.
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683.
- Abdella, S.; Afinjuomo, F.; Song, Y.; Upton, R.; Garg, S. Mucoadhesive Buccal Film of Estradiol for Hormonal Replacement Therapy: Development and In-Vivo Performance Prediction. Pharmaceutics 2022, 14, 542.
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519.
- Borujeni, S.H.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589.
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471.
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336.
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30, 360–367.
- Henry, S.; Samaro, A.; Marchesini, F.H.; Shaqour, B.; Macedo, J.; Vanhoorne, V.; Vervaet, C. Extrusion-based 3D printing of oral solid dosage forms: Material requirements and equipment dependencies. Int. J. Pharm. 2021, 598, 120361.
- Ghanizadeh Tabriz, A.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 120147.
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206.
- Fanous, M.; Gold, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Development of immediate release (IR) 3D-printed oral dosage forms with focus on industrial relevance. Eur. J. Pharm. Sci. 2020, 155, 105558.
- Hartzke, D.; Pössl, A.; Schlupp, P.; Runkel, F.E. Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms. Pharmaceutics 2022, 14, 2103.
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78.
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-II: Cellulosic polymers. J. Excip. Food Chem. 2014, 5, 46–55.
- Rials, T.G.; Glasser, W.G. Thermal and dynamic mechanical properties of hydroxypropyl cellulose films. J. Appl. Polym. Sci. 1988, 36, 749–758.
- Picker-Freyer, K.M.; Dürig, T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS PharmSciTech 2007, 8, 92.
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070.
- Pinto, E.; Dürig, T. Cellulose Ethers for Extrusion Applications. In Melt Extrusion: Materials, Technology and Drug Product Design; Repka, M.A., Langley, N., DiNunzio, J., Eds.; Springer: New York, NY, USA, 2013; pp. 123–144.
- Gospodinova, A.; Nankov, V.; Tomov, S.; Redzheb, M.; Petrov, P.D. Extrusion bioprinting of hydroxyethylcellulose-based bioink for cervical tumor model. Carbohydr. Polym. 2021, 260, 117793.
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883.
- Luo, J.; Xia, G.; Liu, L.; Ji, A.; Luo, Q. Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis. Foods 2022, 11, 3286.
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769.
- Rekhi, G.S.; Jambhekar, S.S. Ethylcellulose—A Polymer Review. Drug Dev. Ind. Pharm. 1995, 21, 61–77.
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52.
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148.
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315.
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18.
- Adams, D.; Ounaies, Z.; Basak, A. Printability Assessment of Ethyl Cellulose Biopolymer Using Direct Ink Writing. JOM 2021, 73, 3761–3770.
- Kavimughil, M.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Effect of material composition and 3D printing temperature on hot-melt extrusion of ethyl cellulose based medium chain triglyceride oleogel. J. Food Eng. 2022, 329, 111055.
- Yu, D.G.; Yang, X.L.; Huang, W.D.; Liu, J.; Wang, Y.G.; Xu, H. Tablets with material gradients fabricated by three-dimensional printing. J. Pharm. Sci. 2007, 96, 2446–2456.
- Yu, D.G.; Branford-White, C.; Yang, Y.C.; Zhu, L.M.; Welbeck, E.W.; Yang, X.L. A novel fast disintegrating tablet fabricated by three-dimensional printing. Drug Dev. Ind. Pharm. 2009, 35, 1530–1536.
- Yu, D.G.; Branford-White, C.; Ma, Z.H.; Zhu, L.M.; Li, X.Y.; Yang, X.L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 2009, 370, 160–166.
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795.
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses. Polym. J. 1985, 17, 143–152.
- Wen, H.; Morris, K.R.; Park, K. Study on the Interactions Between Polyvinylpyrrolidone (PVP) and Acetaminophen Crystals: Partial Dissolution Pattern Change. J. Pharm. Sci. 2005, 94, 2166–2174.
- Kroschwitz, J. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 1993; Volume 10.
- Dürig, T. Binders in Pharmaceutical Granulation. In Handbook of Pharmaceutical Granulation Technology, 3rd ed.; Parikh, Ed.; Informa Health Care: New York, NY, USA, 2010; pp. 78–97.
- Van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269.
- Fikentscher, H.; Herrle, K. Polyvinylpyrrolidone. Mod. Plast. 1946, 23, 157–161, 212, 214, 216, 218.
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2005.
- Rahman, M.; Ozkan, S.; Lester, J.; Farzana, I.; Bi, V.; Dürig, T. Plasticizer Compatibility and thermal and rheological properties of Plasdone povidone and copovidone polymers for hot-melt extrusion applications. In Proceedings of the Annual Meeting of the American Association of Pharmaceutical Scientists (AAPS), Chicago, IL, USA, 14–18 October 2012.
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152.
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368.
- Lee, K.-J.; Kang, A.; Delfino, J.J.; West, T.G.; Chetty, D.; Monkhouse, D.C.; Yoo, J. Evaluation of Critical Formulation Factors in the Development of a Rapidly Dispersing Captopril Oral Dosage Form. Drug Dev. Ind. Pharm. 2003, 29, 967–979.
- Fitzgerald, S. FDA Approves First 3D-printed epilepsy drug experts assess the benefits and caveats. Neurol. Today 2015, 15, 26–27.
- Tian, P.; Yang, F.; Yu, L.-P.; Lin, M.-M.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Applications of excipients in the field of 3D printed pharmaceuticals. Drug Dev. Ind. Pharm. 2019, 45, 905–913.
- Sen, K.; Mukherjee, R.; Sansare, S.; Halder, A.; Kashi, H.; Ma, A.W.K.; Chaudhuri, B. Impact of powder-binder interactions on 3D printability of pharmaceutical tablets using drop test methodology. Eur. J. Pharm. Sci. 2021, 160, 105755.
- Tian, P.; Yang, F.; Xu, Y.; Lin, M.-M.; Yu, L.-P.; Lin, W.; Lin, Q.-F.; Lv, Z.-F.; Huang, S.-Y.; Chen, Y.-Z. Oral disintegrating patient-tailored tablets of warfarin sodium produced by 3D printing. Drug Dev. Ind. Pharm. 2018, 44, 1918–1923.
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder jetting 3D printing of challenging medicines: From low dose tablets to hydrophobic molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159.
- Dores, F.; Kuźmińska, M.; Soares, C.; Bohus, M.; A Shervington, L.; Habashy, R.; Pereira, B.C.; Peak, M.; Isreb, A.; Alhnan, M.A. Temperature and solvent facilitated extrusion based 3D printing for pharmaceuticals. Eur. J. Pharm. Sci. 2020, 152, 105430.
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314.
- Butreddy, A.; Sarabu, S.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Bi, V.; Durig, T.; Repka, M.A. Influence of Plasdone(™) S630 Ultra-an Improved Copovidone on the Processability and Oxidative Degradation of Quetiapine Fumarate Amorphous Solid Dispersions Prepared via Hot-Melt Extrusion Technique. AAPS PharmSciTech 2021, 22, 196.
- Arndt, O.-R.; Kleinebudde, P. Influence of binder properties on dry granules and tablets. Powder Technol. 2018, 337, 68–77.
- Bühler, V. Kollidon-Polyvinylpyrrolidone Excipients for the Pharmaceutical Industry; BASF SE Pharma Ingredients & Services: Ludwigshafen, Germany, 2008.
- Parikh, T.; Gupta, S.S.; Meena, A.K.; Vitez, I.; Mahajan, N.; Serajuddin, A.T. Application of film-casting technique to investigate drug-polymer miscibility in solid dispersion and hot-melt extrudate. J. Pharm. Sci. 2015, 104, 2142–2152.
- Moroni, A. A Novel Copovidone Binder for Dry Granulation and Direct-Compression Tableting. Pharm. Technol. 2001, 1, 8.
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401.
- Boniatti, J.; Januskaite, P.; Fonseca, L.B.D.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114.
- Chang, S.-Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063.
- Gottschalk, N.; Burkard, A.; Quodbach, J.; Bogdahn, M. Drop-on-powder 3D printing of amorphous high dose oral dosage forms: Process development, opportunities and printing limitations. Int. J. Pharm. X 2023, 5, 100151.
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107.
- Barakh Ali, S.F.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651.
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110.
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172.
- Mohamed, E.M.; Barakh Ali, S.F.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation Optimization of Selective Laser Sintering 3D-Printed Tablets of Clindamycin Palmitate Hydrochloride by Response Surface Methodology. AAPS PharmSciTech 2020, 21, 232.
- Davis, D.A.; Thakkar, R.; Su, Y.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J. Pharm. Sci. 2021, 110, 1432–1443.
- Duffy, P.; McMahon, S.; Wang, X.; Keaveney, S.; O’Cearbhaill, E.D.; Quintana, I.; Rodríguez, F.J.; Wang, W. Synthetic bioresorbable poly-α-hydroxyesters as peripheral nerve guidance conduits; a review of material properties, design strategies and their efficacy to date. Biomater. Sci. 2019, 7, 4912–4943.
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58.
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymer 2011, 3, 1377–1397.
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392.
- Agrawal, C.; Niederauer, G.; Micallef, D.; Athanasiou, K. The use of PLA-PGA polymers in orthopaedics. Encycl. Handb. Biomater. Bioeng. Part A Mater. 1995, 2, 1055.
- Löfgren, A.; Albertsson, A.-C.; Dubois, P.; Jérôme, R. Recent Advances in Ring-Opening Polymerization of Lactones and Related Compounds. J. Macromol. Sci. Part C 1995, 35, 379–418.
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076.
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133.
- Nguyen, T.Q.; Kausch, H.H. Molecular Weight Distribution and Mechanical Properties. In Mechanical Properties and Testing of Polymers: An A–Z Reference; Springer Netherlands: Dordrecht, The Netherlands, 1999; pp. 143–150.
More
