HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase inhibitors, commonly known as statins, are the primary treatment choice for cardiovascular diseases, which stand as the leading global cause of mortality. Statins also offer various pleiotropic effects, including improved endothelial function, anti-inflammatory properties, reduced oxidative stress, anti-thrombotic effects, and the stabilization of atherosclerotic plaques. However, the usage of statins can be accompanied by a range of adverse effects, such as the development of type 2 diabetes mellitus, muscular symptoms, liver toxicity, kidney diseases, cataracts, hemorrhagic strokes, and psychiatric complications. These issues are referred to as statin-associated symptoms (SAS) and are relatively infrequent in clinical trials, making it challenging to attribute them to statin use definitively. Therefore, these symptoms can lead to significant problems, necessitating dose adjustments or discontinuation of statin therapy.
- statin
- pleiotropic effects
- statin-associated symptoms
1. Introduction
2. Pleiotropic Benefits of Statin
2.1. Enhancing Endothelial Function
The onset of atherosclerosis often involves endothelial dysfunction, which is influenced by established cardiovascular risk factors such as hypertension, smoking, and high blood sugar levels. These factors, mediated by nitric oxide (NO), can interfere with the regular vasodilation process. Statins play a role in inhibiting the prenylation of Rac and Rho proteins, leading to an increase in the production of endothelium-derived nitric oxide synthetase (eNOS). This, in turn, enhances the production of nitric oxide and supports vasodilation [36][23]. This increased eNOS expression results in the elevated production of nitric oxide within the endothelium, which supports vasodilation [37,38][24][25]. NO release serves not only to facilitate vasodilation but also to hinder leukocyte adhesion, prevent platelet aggregation, and reduce the proliferation of vascular smooth muscle. As a result, NO plays a protective role, and inadequate levels indicate a greater risk of cardiovascular events [39][26]. A schematic representation in Figure 31 shows alterations in the vessel wall and endothelial cell membrane that occur during hyperlipidemia. These changes include LDL introduction, monocyte adhesion, platelet aggregation, foam cell formation, and the accumulation of cholesterol crystals in the intima. Alterations in the endothelial cell membrane include the presence of cholesterol-rich domains and increased caveolae, where caveolin plays a key role in regulating eNOS. These modifications are attenuated by HMG-CoA reductase inhibitors, which lower LDL levels and inhibit cholesterol and isoprenoid biosynthesis pathways [38][25].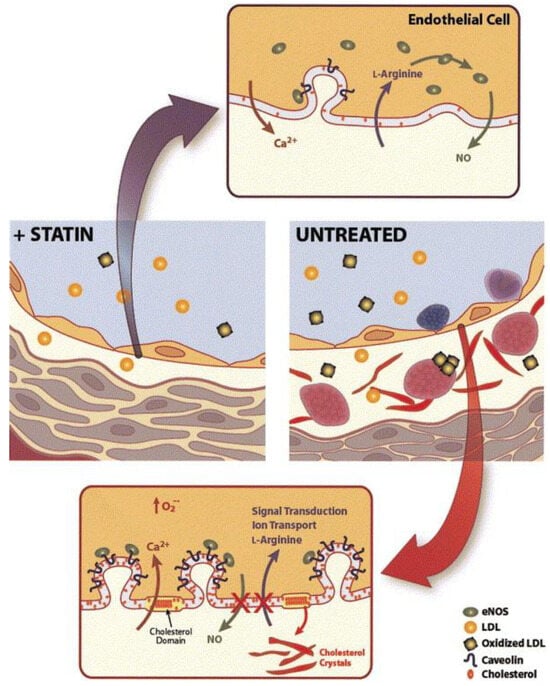
2.2. Plaque Stabilization
2.2. Plaque Stabilization
2.3. Anti-Inflammatory Effects
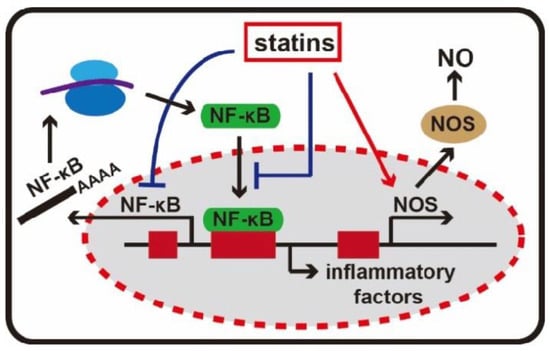
2.4. Immunomodulatory Effects
There is informal evidence to suggest that statins may possess anti-inflammatory and immunomodulatory properties, which could be beneficial in conditions such as cardiac transplant rejection and various autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, lupus, vasculitis, and systemic sclerosis [51]. Statins inhibit the induction of MHC-I2.5. Anti-Thrombotic Effects
2.5. Anti-Thrombotic Effects
In ththe final stage of atherosclerosis, damage to the e final stage of atherosclerosis, damage to the endothedothelium can lead to the formation of blood clots that obstruct blood flow. Statins can impede the formation of blood clots through multiple mechanisms, including a reduction in tissue factor expression and the inhibition of platelet aggregation [55,56,57][41][42][43]. This results in a decrease in thrombin production and the expression of its receptor on platelet surfaces. In addition to preventing blood clot formation, statins also promote the dissolution of clots by reducing levels of plasminogen activator inhibitor 1 (PA1-1) and enhancing the activity of the fibrinolytic enzyme plasminogen. The anticoagulant properties of statins were demonstrated in the JUPITER study, which showed a decreased rate of peripheral venous thromboembolism in patients taking rosuvastatin [58,59][44][45].2.6. Reduced Oxidative Stress
2.7. Protection from High-Decibel Noise-Inducing Hearing Loss
In a recent study, researchers evaluated the potential of statins as a treatment for hearing loss in CBA/CaJ mice [64][50]. They investigated the effects of delivering fluvastatin directly to the cochlea and administering lovastatin orally, assessing hearing outcomes using methods such as auditory brain stem responses (ABRs). Mice were exposed to two hours of octave band noise. Previous research with guinea pigs had demonstrated the protective effects of fluvastatin in the contralateral cochlea. Exposure to high-intensity noise (120 dB SPL for 4 h at 4–8 kHz) was found to result in the loss of hair cells—a phenomenon absent in guinea pigs subjected to noise but treated with fluvastatin. [65][51].2.8. Enhance Responses to Immune Checkpoint Blockade in Cancer Models
In the early stages of statin research, concerns arose about a potential link between statin use and cancer risk, particularly with lipophilic statins such as simvastatin [66][52]. Early observational studies suggested a link between statins and an increased risk of certain cancers [67][53]. This concern originated from the understanding that statins, acting as cholesterol-lowering agents, influence cellular processes associated with cancer development. The reduction in cholesterol, crucial for cell membrane integrity, raised theoretical concerns about long-term consequences. However, as more robust studies unfolded, these early apprehensions lacked consistent support.3. Adverse Effects of Statin Therapy
Statin therapy has frequently been associated with several unintended adverse effects, which further contribute to the concept of statin pleiotropy (Figure 63).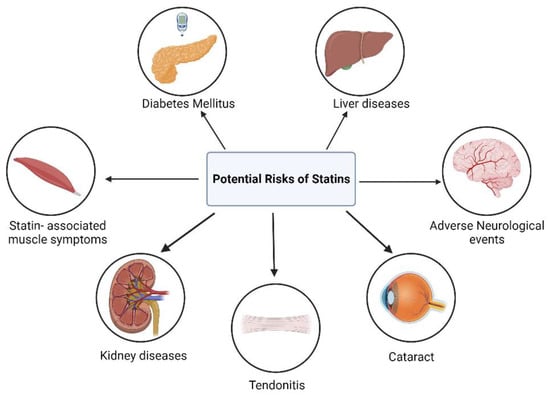
3.1. Myopathy and Rhabdomyolysis
The most common side effect associated with statin use is muscle-related symptoms. Myopathy is typically characterized by muscle pain, tenderness, or weakness, accompanied by a significant increase in blood creatine kinase (CK) levels, which frequently exceed ten times the upper limit of normal (ULN) in laboratory tests. Creatine kinase is an enzyme released when muscle cells are damaged. Rhabdomyolysis is a severe form of myopathy where CK levels exceed 40 times the ULN. This condition involves the breakdown of muscle tissue, releasing myoglobin into the bloodstream. This can potentially result in sudden kidney failure or impaired renal function. This condition is characterized by significantly higher elevations in creatine kinase levels [74,75][54][55]. Preclinical research suggests that statins can reduce mitochondrial activity, lower energy production, and impact muscle protein breakdown, potentially linking statin use to muscle-related symptoms [76][56]. Clinical and scientific studies have been used to investigate the mechanisms underlying muscular side effects associated with statin therapy [77][57]. Muscle biopsies of statin-treated patients with normal creatine kinase (CK) levels revealed mitochondrial dysfunction, lipid accumulation, and structural changes.3.2. Diabetes Mellitus
Data from RCTs show an increased incidence of diabetes mellitus during statin therapy is due to the patients who are already at a higher risk of diabetes progressing to diabetes earlier than they would have otherwise [83,84][58][59]. Patients develop chronic insulin resistance and experience a progressive loss of beta-cell function over an extended period of time, leading to the development of type 2 diabetes mellitus [85][60]. The JUPITER trial observed elevated levels of glycated hemoglobin in individuals taking rosuvastatin, along with a slight increase in the occurrence of diabetes mellitus (3.0% vs. 2.4%, p = 0.01) compared with those on placebo [58,86][44][61].3.3. Liver Diseases
The research on the effects of statins on liver disease has demonstrated both potential benefits and concerns. On the positive side, statins have been associated with improvements in liver enzyme levels and a potential reduction in the progression of non-alcoholic fatty liver disease (NAFLD) [88][62]. On the contrary, initial clinical trials of statins observed increased aminotransferase levels in around 2% of patients. A common side effect, often resolving when the dosage is reduced, is the asymptomatic elevation of hepatic enzyme activity [89][63]. Despite their widespread use worldwide, acute liver failure has been rare. However, Statin-induced drug-induced liver injury (DILI) causing acute liver failure (ALF) remains a concern [90][64].3.4. Adverse Neurological Events
Neurological conditions associated with statin use include hemorrhagic stroke, cognitive decline, peripheral neuropathy, depression, memory issues, aggression, and personality changes [92][65]. The impact of statins on intracerebral hemorrhages (ICH) has been debated, with some studies suggesting a potential risk increase [31][66], while recent comprehensive research and meta-analyses did not find a clear association between statin use and ICH [93,94,95][67][68][69]. The impact of statin medication on memory loss has been investigated with varying findings. Some studies suggest that statin use could lower the risk of cognitive decline or dementia, including Alzheimer’s disease. These potential neuroprotective effects are hypothesized to be related to statins’ anti-inflammatory and antioxidant properties, which may benefit brain health. However, in some studies, no compelling evidence was discovered indicating a connection between statins and Alzheimer’s disease or cognitive function [96,97][70][71].3.5. Cataract
Observational data, along with limited preclinical research, suggested a potential link between the use of statins and the development of cataracts. In a clinical investigation, the researchers highlighted that triparanol could trigger cataracts in both white rats and human subjects. As a result of the occurrence of cataracts and other side effects in individuals in various age groups, the therapeutic application of triparanol was discontinued [99][72].3.6. Kidney Diseases
According to a study conducted by the Canadian Network for Observational Drug Effect Studies (CNODES), individuals taking high-potency statins, as opposed to low-potency ones, faced a 34% higher risk of being hospitalized for acute kidney injury (AKI) within 120 days of starting treatment [102][73]. In the first two years of commencing lower-dose statin medication, approximately 1 in 500 patients needed hospitalization for AKI. For those using more potent statins during the same period, there was a 15% higher relative risk of experiencing renal damage [102][73].3.7. Tendonitis and Tendon Rupture
According to numerous studies and case reports, statins may increase the risk of tendon rupture [105][74]. In a case-control study conducted, exposure to statins was compared between 93 cases of tendon rupture and 279 sex- and age-matched controls. There was no significant difference in statin use rates between cases and controls. However, subgroup analysis revealed that statin exposure was a significant risk factor for tendon rupture in women but not in men [106][75].References
- Cardiovascular Diseases (CVDs); World Health Organization: Geneva, Switzerland, 2021.
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639.
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492.
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; STOKES, J., III. Factors of risk in the development of coronary heart disease—Six-year follow-up experience: The Framingham Study. Ann. Intern. Med. 1961, 55, 33–50.
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478.
- Gofman, J.W.; Delalla, O.; Glazier, F.; Freeman, N.K.; Lindgren, F.T.; Nichols, A.V.; Strisower, B.; Tamplin, A.R. The serum lipoprotein transport system in health, metabolic disorders, atherosclerosis and coronary heart disease. J. Clin. Lipidol. 2007, 1, 104–141.
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533.
- Vega, G.L.; Grundy, S.M. Influence of lovastatin therapy on metabolism of low density lipoproteins in mixed hyperlipidaemia. J. Intern. Med. 1991, 230, 341–350.
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172.
- Goldstein, J.L.; Brown, M.S. Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl. Acad. Sci. USA 1973, 70, 2804–2808.
- Manzoni, M.; Rollini, M. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 2002, 58, 555–564.
- Xie, X.; Tang, Y. Efficient synthesis of simvastatin by use of whole-cell biocatalysis. Appl. Environ. Microbiol. 2007, 73, 2054–2060.
- Murphy, C.; Deplazes, E.; Cranfield, C.G.; Garcia, A. The role of structure and biophysical properties in the pleiotropic effects of statins. Int. J. Mol. Sci. 2020, 21, 8745.
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164.
- Ziaeian, B.; Fonarow, G.C. Statins and the prevention of heart disease. JAMA Cardiol. 2017, 2, 464.
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.; Keech, A.; Simes, J. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet 2010, 376, 1670–1681.
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278.
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto Jr, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207.
- Ridker, P.M.; Rifai, N.; Pfeffer, M.A.; Sacks, F.M.; Moye, L.A.; Goldman, S.; Flaker, G.C.; Braunwald, E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation 1998, 98, 839–844.
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209.
- Muscoli, S.; Ifrim, M.; Russo, M.; Candido, F.; Sanseviero, A.; Milite, M.; Di Luozzo, M.; Marchei, M.; Sangiorgi, G.M. Current Options and Future Perspectives in the Treatment of Dyslipidemia. J. Clin. Med. 2022, 11, 4716.
- Kosmas, C.E.; Pantou, D.; Sourlas, A.; Papakonstantinou, E.J.; Uceta, R.E.; Guzman, E. New and emerging lipid-modifying drugs to lower LDL cholesterol. Drugs Context 2021, 10, 1–22.
- Laufs, U.; La Fata, V.; Plutzky, J.; Liao, J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998, 97, 1129–1135.
- Ota, H.; Eto, M.; Kano, M.R.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2205–2211.
- Dichtl, W.; Dulak, J.; Frick, M.; Alber, H.F.; Schwarzacher, S.P.; Ares, M.P.; Nilsson, J.; Pachinger, O.; Weidinger, F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 58–63.
- Kuwahara, N.; Sasaki, S.; Kobara, M.; Nakata, T.; Tatsumi, T.; Irie, H.; Narumiya, H.; Hatta, T.; Takeda, K.; Matsubara, H. HMG-CoA reductase inhibition improves anti-aging klotho protein expression and arteriosclerosis in rats with chronic inhibition of nitric oxide synthesis. Int. J. Cardiol. 2008, 123, 84–90.
- Mason, R.P.; Walter, M.F.; Jacob, R.F. Effects of HMG-CoA reductase inhibitors on endothelial function: Role of microdomains and oxidative stress. Circulation 2004, 109, II-34–II-41.
- Hattori, K.; Ozaki, Y.; Ismail, T.F.; Okumura, M.; Naruse, H.; Kan, S.; Ishikawa, M.; Kawai, T.; Ohta, M.; Kawai, H. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter–IVUS. JACC Cardiovasc. Imaging 2012, 5, 169–177.
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080.
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.-C. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 2006, 295, 1556–1565.
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011, 365, 2078–2087.
- Komukai, K.; Kubo, T.; Kitabata, H.; Matsuo, Y.; Ozaki, Y.; Takarada, S.; Okumoto, Y.; Shiono, Y.; Orii, M.; Shimamura, K. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: The EASY-FIT study. J. Am. Coll. Cardiol. 2014, 64, 2207–2217.
- Lee, S.-E.; Chang, H.-J.; Sung, J.M.; Park, H.-B.; Heo, R.; Rizvi, A.; Lin, F.Y.; Kumar, A.; Hadamitzky, M.; Kim, Y.J. Effects of statins on coronary atherosclerotic plaques: The PARADIGM study. JACC Cardiovasc. Imaging 2018, 11, 1475–1484.
- Zhang, Q.; Dong, J.; Yu, Z. Pleiotropic use of Statins as non-lipid-lowering drugs. Int. J. Biol. Sci. 2020, 16, 2704–2711.
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M.; Investigators, P.; Investigators, P. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70.
- Subramanian, S.; Emami, H.; Vucic, E.; Singh, P.; Vijayakumar, J.; Fifer, K.M.; Alon, A.; Shankar, S.S.; Farkouh, M.; Rudd, J.H. High-dose atorvastatin reduces periodontal inflammation: A novel pleiotropic effect of statins. J. Am. Coll. Cardiol. 2013, 62, 2382–2391.
- Wiklund, O.; Mattsson-Hulten, L.; Hurt-Camejo, E.; Oscarsson, J. Effects of simvastatin and atorvastatin on inflammation markers in plasma. J. Intern. Med. 2002, 251, 338–347.
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987.
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402.
- Hakamada-Taguchi, R.; Uehara, Y.; Kuribayashi, K.; Numabe, A.; Saito, K.; Negoro, H.; Fujita, T.; Toyo-oka, T.; Kato, T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ. Res. 2003, 93, 948–956.
- Ali, F.Y.; Armstrong, P.C.; Dhanji, A.-R.A.; Tucker, A.T.; Paul-Clark, M.J.; Mitchell, J.A.; Warner, T.D. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 706–711.
- Haramaki, N.; Ikeda, H.; Takenaka, K.; Katoh, A.; Sugano, R.; Yamagishi, S.-i.; Matsuoka, H.; Imaizumi, T. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: Possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1471–1477.
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103.
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571.
- Joseph, P.; Glynn, R.; Lonn, E.; Ramasundarahettige, C.; Eikelboom, J.; MacFadyen, J.; Ridker, P.; Yusuf, S. Rosuvastatin for the prevention of venous thromboembolism: A pooled analysis of the HOPE-3 and JUPITER randomized controlled trials. Cardiovasc. Res. 2022, 118, 897–903.
- Wassmann, S.; Laufs, U.; Bäumer, A.T.; Müller, K.; Ahlbory, K.; Linz, W.; Itter, G.; Rösen, R.; Böhm, M.; Nickenig, G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension 2001, 37, 1450–1457.
- Aviram, M.; Hussein, O.; Rosenblat, M.; Schlezinger, S.; Hayek, T.; Keidar, S. Interactions of platelets, macrophages, and lipoproteins in hypercholesterolemia: Antiatherogenic effects of HMG-CoA reductase inhibitor therapy. J. Cardiovasc. Pharmacol. 1998, 31, 39–45.
- Wassmann, S.; Laufs, U.; Müller, K.; Konkol, C.; Ahlbory, K.; Bäumer, A.T.; Linz, W.; Böhm, M.; Nickenig, G. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 300–305.
- Takemoto, M.; Node, K.; Nakagami, H.; Liao, Y.; Grimm, M.; Takemoto, Y.; Kitakaze, M.; Liao, J.K. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Investig. 2001, 108, 1429–1437.
- Dépreux, F.; Czech, L.; Young, H.; Richter, C.-P.; Zhou, Y.; Whitlon, D.S. Statins protect mice from high-decibel noise-induced hearing loss. Biomed. Pharmacother. 2023, 163, 114674.
- Richter, C.-P.; Young, H.; Richter, S.V.; Smith-Bronstein, V.; Stock, S.R.; Xiao, X.; Soriano, C.; Whitlon, D.S. Fluvastatin protects cochleae from damage by high-level noise. Sci. Rep. 2018, 8, 3033.
- Matsuzaki, M.; Kita, T.; Mabuchi, H.; Matsuzawa, Y.; Nakaya, N.; Oikawa, S.; Saito, Y.; Sasaki, J.; Shimamoto, K.; Itakura, H. Large Scale Cohort Study of the Relationship between Serum Cholesterol Concentration and Coronary Events with Low-Dose Simvastatin Therapy in Japanese Patients with Hypercholesterolemia Primary Prevention Cohort Study of the Japan Lipid Intervention Trial (J-LIT). Circ. J. 2002, 66, 1087–1095.
- Newman, T.B.; Hulley, S.B. Carcinogenicity of lipid-lowering drugs. JAMA 1996, 275, 55–60.
- Law, M.; Rudnicka, A.R. Statin safety: A systematic review. Am. J. Cardiol. 2006, 97, S52–S60.
- Cheeley, M.K.; Saseen, J.J.; Agarwala, A.; Ravilla, S.; Ciffone, N.; Jacobson, T.A.; Dixon, D.L.; Maki, K.C. NLA scientific statement on statin intolerance: A new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J. Clin. Lipidol. 2022, 16, 361–375.
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, L.; Nordestgaard, B.G. Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur. Heart J. 2015, 36, 1012–1022.
- Ruscica, M.; Reiner, Z.; Sirtori, C.R. Can we further optimize statin therapy to increase tolerability? Expert. Opin. Drug Discov. 2019, 14, 843–847.
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742.
- Rajpathak, S.N.; Kumbhani, D.J.; Crandall, J.; Barzilai, N.; Alderman, M.; Ridker, P.M. Statin therapy and risk of developing type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 1924–1929.
- Chogtu, B.; Magazine, R.; Bairy, K. Statin use and risk of diabetes mellitus. World J. Diabetes 2015, 6, 352.
- Miller, P.E.; Martin, S.S. Approach to statin use in 2016: An update. Curr. Atheroscler. Rep. 2016, 18, 20.
- Pastori, D.; Pani, A.; Di Rocco, A.; Menichelli, D.; Gazzaniga, G.; Farcomeni, A.; D’Erasmo, L.; Angelico, F.; Del Ben, M.; Baratta, F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 2022, 88, 441–451.
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin toxicity: Mechanistic insights and clinical implications. Circ. Res. 2019, 124, 328–350.
- Reuben, A.; Koch, D.G.; Lee, W.M. Drug-induced acute liver failure: Results of a US multicenter, prospective study. Hepatology 2010, 52, 2065–2076.
- Vergouwen, M.D.; De Haan, R.J.; Vermeulen, M.; Roos, Y.B. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke 2008, 39, 497–502.
- Amarenco, P. For the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators: High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559.
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond lipid-lowering: Effects of statins on cardiovascular and cerebrovascular diseases and cancer. Pharmaceuticals 2022, 15, 151.
- Ribe, A.R.; Vestergaard, C.H.; Vestergaard, M.; Pedersen, H.S.; Prior, A.; Lietzen, L.W.; Brynningsen, P.K.; Fenger-Grøn, M. Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke 2020, 51, 1111–1119.
- Hackam, D.G.; Woodward, M.; Newby, L.K.; Bhatt, D.L.; Shao, M.; Smith, E.E.; Donner, A.; Mamdani, M.; Douketis, J.D.; Arima, H. Statins and intracerebral hemorrhage: Collaborative systematic review and meta-analysis. Circulation 2011, 124, 2233–2242.
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and cognition: A systematic review and meta-analysis of short-and long-term cognitive effects. Mayo Clin. Proc. 2013, 88, 1213–1221.
- Richardson, K.; Schoen, M.; French, B.; Umscheid, C.A.; Mitchell, M.D.; Arnold, S.E.; Heidenreich, P.A.; Rader, D.J.; Degoma, E.M. Statins and cognitive function: A systematic review. Ann. Intern. Med. 2013, 159, 688–697.
- Kirby, T. Cataracts produced by triparanol.(MER-29). Trans. Am. Ophthalmol. Soc. 1967, 65, 494.
- Dormuth, C.R.; Hemmelgarn, B.R.; Paterson, J.M.; James, M.T.; Teare, G.F.; Raymond, C.B.; Lafrance, J.-P.; Levy, A.; Garg, A.X.; Ernst, P. Use of high potency statins and rates of admission for acute kidney injury: Multicenter, retrospective observational analysis of administrative databases. BMJ 2013, 346, f880.
- Kearns, M.C.; Singh, V.K. Bilateral patellar tendon rupture associated with statin use. J. Surg. Case Rep. 2016, 2016, rjw072.
- Beri, A.; Dwamena, F.C.; Dwamena, B.A. Association between statin therapy and tendon rupture: A case-control study. J. Cardiovasc. Pharmacol. 2009, 53, 401–404.
