Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Anwar Abdelnaser.
Multiple sclerosis is the predominant autoimmune disorder affecting the central nervous system in adolescents and adults. Specific treatments are categorized as disease-modifying, whereas others are symptomatic treatments to alleviate painful symptoms. No singular conventional therapy is universally effective for all patients across all stages of the illness. Nevertheless, cannabinoids exhibit significant promise in their capacity for neuroprotection, anti-inflammation, and immunosuppression.
- multiple sclerosis
- autoimmune disease
- cannabinoids
- tetrahydrocannabinol
- cannabis
- treatment modalities
- immunomodulatory
- nanomedicine
1. Introduction
Multiple sclerosis (MS) is an autoimmune disorder that affects the central nervous system (CNS) [1] and is one of the leading causes of neurological impairment in teenagers and adults [2]. Multiple sclerosis can be mainly categorized into three types: Relapsing-Remitting MS (RRMS), Secondary Progressive MS (SPMS), and Primary Progressive MS (PPMS). Most MS patients (85–90%) initially present with RRMS, with around 90% eventually transitioning to SPMS and the remaining 10% experiencing PPMS [3]. Multiple sclerosis (MS) is distinguished by the presence of muscle spasms, spasticity, neuropathic pain, bladder dysfunction, tremors, dysarthria, and cognitive impairments, such as memory disturbances [4]. There is currently a growing trend in utilizing cannabis for therapeutic purposes as a symptomatic treatment. Numerous trials and patients have reported that it may be beneficial in managing and controlling symptoms associated with multiple sclerosis (MS).
2. Cannabinoids and the Endocannabinoid System (ECS)
2.1. Cannabinoids
Cannabis sativa, Cannabis indica, and Cannabis ruderalis are the three most common species of the cannabis plant, which is in the Cannabaceae family [32][5]. The cannabis plant has a long history of practical uses, including as a food and oil source and even in producing paper and linen, two of man’s necessities [33][6]. In addition, its psychoactive qualities enabled its use in medical surgeries, even though its components and mechanism of action in the human body were unknown at the time [34][7]. Phytocannabinoids, endocannabinoids, and synthetic cannabinoids are the three primary sources of more than sixty cannabinoids with physiological effects [8]. The cannabis plant contains more than 100 phytocannabinoids, including the two most significant ones, which are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). It is believed that Δ9-THC is the primary psychoactive compound found in cannabis [9].2.2. The Endocannabinoid System (ECS)
The primary impact of cannabinoids occurs through the endocannabinoid system (ECS), which consists of a set of signaling pathways regulated by cannabinoid receptors cannabinoid-1 (CB1) and cannabinoid-2 (CB2). The activation of these pathways is commonly triggered by the attachment of endogenous cannabinoids (endocannabinoids) like Anandamide (AEA) and 2-Arachidonoyl Glycerol (2-AG) to the CB1 and CB2 receptors [35][10]. The CB1 receptors are situated primarily in nerve terminals and function to inhibit the release of neurotransmitters. Conversely, CB2 receptors are predominantly located in immune cells. Their role encompasses regulating cytokine production and migrating immune cells within and beyond the central nervous system [36,37][11][12]. The endocannabinoid system (ECS) is essential for maintaining the body’s homeostasis by regulating the balance between the inhibitory and excitatory states of the nerves. This is accomplished by activating CB1 receptors located on inhibitory GABAergic and excitatory glutamatergic presynaptic terminals, inhibiting neurotransmitter release [38][13]. Additionally, the ECS is responsible for many physiological and pathological processes in the body. It controls biological mechanisms, such as pain, food intake, anxiety, and memory [39][14].3. Neuroprotection Effect of Cannabinoids
The neuroprotective effect of cannabinoids in multiple sclerosis (MS) may be attributed to their role in regulating the excessive excitability of neurons in the central nervous system (CNS). The CB1 receptor is located predominantly in GABAergic neurons within the hippocampus. It is also found in neurons that use glutamate as a neurotransmitter and in astrocytes and subcellular compartments [40,41][15][16]. The release of cholinergic and dopaminergic neurotransmitters is regulated by cannabinoid signaling and the regulation of excitatory/inhibitory transmission by CB1 receptors, as shown in Figure 1 [42,43][17][18]. Studies have shown that cannabinoid-based therapy can effectively reduce symptoms of multiple sclerosis, such as spasticity, pain, gallbladder dysfunction, and tremors [44][19], which is achieved by increasing the secretion of endocannabinoids in targeted areas, activating CB1 receptors, and limiting the release of neurotransmitters from presynaptic terminals, which results in a reduction in the excessive excitatory state in the neurons and a potential neuroprotection effect of the CNS [45][20]. In addition, cannabinoids’ impact on the regulation and modulation of microglial cells within the CNS has been investigated. Inflammation has been shown to elevate CB2 receptors in glial and immune cells, even though they are less prevalent in the healthy brain, as observed in EAE models [46][21].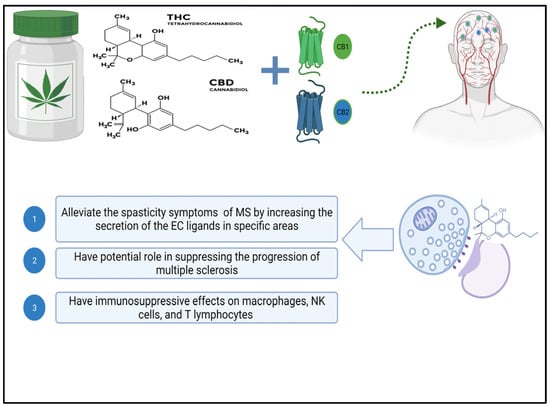
Figure 1. Diagram depicting the biological effects of cannabis’ active ingredients on multiple sclerosis. Abbreviations for cannabinoid receptors 1 and 2, tetrahydrocannabinol, and cannabidiol created with Biorender.
4. Immunomodulatory Effect of Cannabinoids
The presence of cannabinoid receptor 2 (CB2) in white blood cells has sparked interest in the ability of cannabinoids to regulate the immune system. THC binds to CB1 receptors in the brain, whereas CB2 receptors are found predominantly in immune cells in the peripheral nervous system. The precise role of the endocannabinoid system in immune regulation is not yet fully comprehended, despite evidence of cannabinoids affecting immune cell function [49,50][24][25]. According to a study by Nichols et al. in 2020, cannabidiol (CBD) has been recognized as an anti-inflammatory substance and has some characteristics of suppressing the immune system [51][26]. Exposure to high concentrations of cannabis can impair immune responses, according to in vitro and in vivo research. This reduces the activity and cytokine production capacity of macrophages, natural killer cells, and T lymphocytes [52][27]. However, rather than reducing immune system activity, an adequate amount of cannabis in the body increases lymphocyte metabolic activity and boosts the production of pro-inflammatory cytokines [53][28]. These dose-dependent cannabinoid activities point to the biphasic effect of cannabis constituents [52][27]. Despite this potential biphasic effect of cannabinoids, CBD has been shown in several studies to act as an immunomodulator during inflammation, regulating the inflammatory response by influencing various inflammatory cascades involving both anti-inflammatory and pro-inflammatory mediators, as discussed in the study by Furgiuele et al. [54][29]. Inflammation, axonal demyelination, and symptoms like spasticity and pain are all helped by these neuroprotective mechanisms. Using EAE murine models of multiple sclerosis, researchers found that CBD, with the help of myeloid-derived suppressor cells (MDSCs), improved EAE progression dose-dependently. According to the research conducted by Elliott et al., CBD had several effects, including a decrease in T-cell proliferation in the central nervous system (CNS) and a decrease in the pro-inflammatory cytokines IL-17 and IFNγ [55][30]. Additionally, CBD treatment decreased inflammation and axonal loss in multiple sclerosis models engineered with myelin oligodendrocyte glycoprotein (MOG) to imitate EAE. The reason for this was that CBD inhibits the infiltration of T-cells and the activation of microglial cells, as reported in the study by Kozela et al. [56][31].5. Therapeutic Potential of Cannabinoids
Cannabidiol has demonstrated encouraging effects in treating a range of medical conditions. Within the domain of epilepsy therapy, CBD has shown efficacy as an anticonvulsant medication, particularly in the treatment of severe childhood epilepsy syndromes such as Dravet syndrome and Lennox–Gastaut syndrome. Furthermore, CBD has been studied for its potential as an antidepressant, antipsychotic, and anxiolytic agent. Moreover, CBD has demonstrated an anticancer effect. While further research is necessary to comprehend its advantages fully, CBD exhibits promise as a reliable and efficient medication, indicating therapeutic implications for inflammation, neuroprotection, epilepsy, depression, and pain [57,58][32][33]. These findings support the potential therapeutic benefits of cannabinoids in managing neuroinflammation and its impact on MS-related pathology. Research has investigated the potential of cannabinoids to inhibit the progression of multiple sclerosis (MS) and provide neuroprotection in animal models. The results have varied under different experimental conditions, as specified in Table 1, and there have yet to be any human trials conducted with appropriate doses. CBD has demonstrated effectiveness in animal MS models and human cells tested in a laboratory setting. However, its impact on the immune system of MS patients is yet to be observed [59][34]. Individual variance and genetic polymorphism may point to distinct processes or responses to cannabinoids, leading to a reasoned explanation.Table 1.
Clinical studies on the use of cannabinoids for the management of multiple sclerosis.
| Treatment Used | Experimental Design | Results | Ref. |
|---|---|---|---|
| A daily dose of CBD (75 mg/kg) for 5 days |
In vivo (mice) | Dimension in the T-cell infiltration and neuroinflammation in the brain and spinal cord’s white matter pathways. | [60][35] |
| A daily dose of CBD (10 mg/kg/day i.p.) | In vivo (mice) | Decrease the proliferation of T-cells. | [61][36] |
| A daily dose of CBD + THC (10 mg/kg/day i.p.) | In vivo (mice) | (CBD + 9-THC) Decrease the number of CD3+ T-cells, CD3+ CD4+ T-cells, and demyelination. Furthermore, the combination of THC and CBD improves the clinical symptoms of MS patients. | [61][36] |
| A daily dose of CBD (20 mg/kg/day i.p.) | In vivo (mice) | Clinical symptoms have a delayed onset and are less severe. | [55][30] |
| Determination of T-cells in marijuana smokers | In vivo (human subjects) | Reduction in the T-cells proliferation. | [50][25] |
| A dose of 10−5 to 10−4 of delta 8-THC + delta 9- THC + CBD | In vitro (Animal cell culture) | Decrease the proliferation of T-cells. | [62][37] |
| Three intraperitoneal (i.p.) injections of CBD (5 mg/kg, one per day) given at the outset of clinical disease | In vitro (cell culture, T-cell line) | Reduction in disease symptoms during the days following the injections, as well as a significant delay in disease development. Additionally, prevention of T-cell proliferation. | [56][31] |
| A dose (10/100 ng/mL) of Δ-THC | In vitro (B cells) | Increase the proliferation of T-cells. | [63][38] |
| After MS induction, CBD (10 mg/kg/day i.p.) was given for 7 days | In vitro (CD4+ T lymphocytes) | CD4+ T-cells’ pro-inflammatory phenotype is reversed. | [64][39] |
| A dose of CBD (0.1–1.5 μM) | In vitro (T-cell line derived from lymph node cells) | CD4+ T-cells and CD19+ B cells succumbed more frequently, whereas CD11b+ monocytes did not. | [65][40] |
| CBD (5–10 mg/kg/3 times per week or 50 mg/kg/day i.p.) | In vivo (mice) | Clinical symptoms and tissue lesions are reduced dose-dependently. | [66][41] |
| A dose (5–10 μg/mL) of THC | In vitro (cell culture) | Decrease in the number of Natural killer cell (NK). | [50][25] |
| Dose of 8 μg/mL or 2.6 × 10−5 M of THC | In vitro (animal cell culture) | Inhibition of T-cell proliferation. | [67][42] |
| 10−5 to 10−4 M concentrations of delta8, and delta9-tetrahydrocannabinol (THC) | In vitro (human cell culture) | Reduction in the proliferation of T-cells. | [62][37] |
6. Nanomedicine and Cannabinoids for MS Treatment
Nanotechnology in drug delivery (nanomedicine) offers the advantage of directing the active components of the medication to specific target areas, resulting in sustained release and improved treatment outcomes [96][43]. Nanomedicine has shown the potential to overcome the limitations of conventional medicines. Utilizing nanotechnology allows for targeted release and precise control over the dosage, leading to improved treatment efficacy and decreased toxicity. Nanomaterials in therapeutic systems have become a promising approach for treating MS, offering both neuroprotection and increased effectiveness by crossing the blood-brain barrier (BBB) [97][44].
6.1. Enhancing Drug Stability and Solubility through Nanomedicine
Nanomedicine greatly influences the chemical properties of loaded drugs, particularly in terms of their stability and solubility. Nanosuspensions and nanotechnology have been demonstrated to augment the solubility and stability of drugs in drug delivery systems, consequently enhancing their bioavailability and bioactivity. Nanoparticles can alter the pharmacokinetics of drugs, leading to improved drug safety and efficacy. Additionally, nanomedicine can address issues such as poor aqueous solubility, poor permeation, low systemic availability, and instability of drugs [98,99,100,101,102][45][46][47][48][49]. For instance, the administration of nanosuspension drugs with low water solubility is an advancing and swiftly expanding domain, garnering heightened interest for its potential to mitigate toxicity and enhance drug effectiveness by eliminating the need for co-solvents in the formulation [103,104][50][51].
Moreover, notable progress has been made in nanomedicine, particularly in improving nanoformulations’ stability using different stabilizers. A comprehensive review by Wu et al. underscored drug nanoparticles’ physical and chemical stability, elucidating the mechanisms and corresponding characterization techniques crucial for sustaining their stability. This advancement significantly enhances the advantages of employing nanotechnology in drug delivery [102][49]. This reformulation of pre-existing medicines or the development of new ones has been substantially boosted by the increasing research in nanomedicine, leading to changes in drug toxicity, solubility, and bioavailability profiles [100,101][47][48]. In conclusion, nanomedicine is pivotal in bolstering the stability and solubility of encapsulated drugs, ultimately enhancing their overall efficacy and safety.
6.2. Mechanism of Nano-Cannabinoids Evading the Blood-Brain Barrier
The blood-brain barrier (BBB) comprises a specialized network of endothelial cells, pericytes, and astrocytes, acting as a defense mechanism to prevent the extravasation of materials. Tight junctions between adjacent brain endothelial cells limit paracellular transport, restricting the passive entry of molecules to a narrow range of size and lipophilicity. These formidable physical and functional barriers impede the exposure of drugs to intracranial tissues. Tight junctions commonly exclude hydrophilic small molecules from entering the brain, and although many lipophilic drugs can passively diffuse, their penetration into diseased brain tissue is often inefficient. This inefficiency typically necessitates high drug doses, leading to dose-limiting systemic toxicity [105,106][52][53]. In light of the numerous challenges associated with the passage of small molecules through the blood-brain barrier, nanoparticles have been investigated as a potential means to enhance drug delivery to brain tissues [107][54]. Much of the current research has concentrated on improving the passive transport mechanisms of drug-loaded nanoparticles across the BBB. For example, in diseases where the BBB is compromised, such as glioblastoma, nanostructures have been observed to extravasate through leaky vasculature, accumulating at tumor sites.
Similarly, strategies have been developed to enhance drug delivery across an intact BBB by initially disrupting this barrier [108,109,110,111][55][56][57][58]. However, such approaches, while allowing unregulated passage across the BBB, may compromise the BBB’s homeostatic functions and expose the brain to harmful toxins and pathogens [112][59]. In contrast, alternative approaches for diseases like SHH subgroup medulloblastoma, where the BBB remains intact, involve the use of nontargeting nanocarriers to prolong the systemic circulation of small-molecule drugs, and over a relatively long time, an appreciable number of drugs would cross the BBB. Nevertheless, this has only partially improved on-target toxicity profiles at high doses [112][59]. Notably, recent research suggests that the passive entry of nanoparticles into solid tumors through gaps between endothelial cells is a minor mechanism, with up to 97% of transport occurring through an active process across endothelial cells [113][60]. A recent study by Tylawsky et al. focused on exploring transendothelial transport facilitated by caveolin-1-dependent transcytosis using nanoparticles that specifically target P-selectin receptors on endothelial cells. This transport mode occurs through brain endothelial cells that maintain an intact blood-brain barrier (BBB). Given these findings, an alternative approach involves leveraging a precise receptor–ligand interaction to enable targeted and controlled delivery of therapeutic agents encapsulated within nanoparticles across an intact BBB [114][61]. Regarding cannabinoids, high levels of P-glycoprotein (P-gp) may reduce the brain delivery of cannabinoids, but decreasing P-gp activity could cause cerebral accumulation. These findings suggest that the use of nanoparticles targeting specific proteins to enhance transcytosis while modulating P-gp activity could potentially facilitate the delivery of nano-cannabinoids across the blood-brain barrier, offering a promising avenue for the treatment of central nervous system disorders [114,115][61][62].
6.3. Nano-Cannabinoids: Challenges and Potentials
Currently, there is no single conventional treatment that works for all MS patients at all stages, and the limited use of FDA-approved cannabinoids in medical practice is due to the inconsistent efficacy, inadequate targeting, and the absence of a thorough understanding of the stability of commercially available cannabis formulations [96,116,117][43][63][64]. Cannabinoids, being lipophilic, have varying oral absorption and distribution levels [118][65]. Additionally, fluctuations in temperature and exposure to light can result in the rapid degradation of cannabinoids [119][66]. The limited bioavailability of cannabinoids is attributed to low absorption rates of 20 to 30% for oral administration and 10–to 60% when inhaled, increasing the need for higher dosages, which may result in toxicity, especially for normal non-targeted tissues. Cannabinoids are also prone to auto-oxidation and degradation, influenced by factors such as light or temperature. All these factors hinder the widespread use of cannabinoid formulations in MS [120,121][67][68]. However, scientists have attempted to overcome the limitations of traditional cannabinoid treatments by incorporating them into nano-based therapeutic systems. This integration aims to improve the stability of cannabinoids, reduce the required dosage for MS treatment, and evade the BBB, thereby targeting MS lesions with minimal toxicity and fewer side effects for normal neural tissues and cells [122][69]. Several studies have formulated various nano-cannabinoid types, including lipid nanoparticles, micelles, silica nanoparticles, and carbon nanotubes [123][70]. This is depicted in Figure 2.
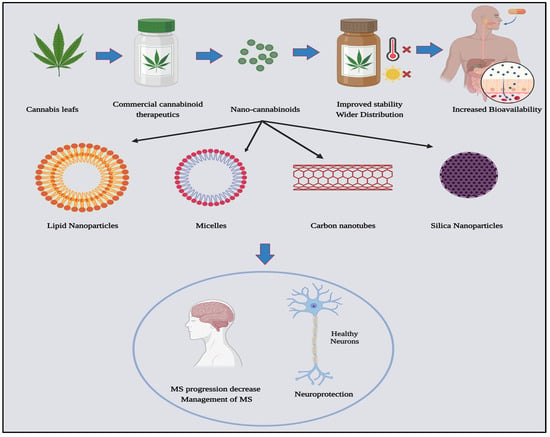

Figure 2.
Nano-cannabinoids: types and potentials in medicine, created with Biorender.
A study by Aparicio-Blanco et al. found that cannabinoid-based nanomaterials and formulations have demonstrated their efficacy against glioma cells. The researchers effectively integrated CBD, a significant compound found in cannabis, into Solid Lipid Nanocapsules (LNCs) through two methods, firstly, by incorporating CBD into the core of the LNCs, and secondly, by decorating the surface of the LNCs with CBD. Both techniques resulted in a slower, sustained release of CBD, decreased the required IC50 for treatment, and effectively targeted glioma cells [124][71]. Moreover, Δ9-tetrahydrocannabinol (Δ9-THC) is recognized for its antitumor activity. Recently, Duran-Lobato et al. conducted a noteworthy study in which they created and evaluated nanoparticles encapsulated with Δ9-THC and targeted to cannabinoid receptors [125][72]. Surprisingly, their technique demonstrated more potential for controlling anticancer effects and the sustained release of cannabinoids at target cells. Recently, multiple studies have shown that encapsulating lipophilic compounds like cannabinoids in nanostructured delivery systems can effectively cross the blood-brain barrier (BBB) with improved targeting ability to the CNS [125][72]. This increases the bioavailability, solubility, and stability of the drug in the body over a more extended period [126,127][73][74]. While cannabinoid-based nanomedicine holds numerous advantages, it has not yet achieved widespread acceptance as a fully developed treatment for patients with multiple sclerosis. There is a crucial need for additional research to further explore and understand its potential benefits and effectiveness, particularly in the context of multiple sclerosis.
References
- Rouleau, I.; Dagenais, E.; Tremblay, A.; Demers, M.; Roger, É.; Jobin, C.; Duquette, P. Prospective memory impairment in multiple sclerosis: A review. Prospect. Mem. Clin. Popul. 2017, 32, 922–936.
- Gerhard, L.; Dorstyn, D.S.; Murphy, G.; Roberts, R.M. Neurological, physical and sociodemographic correlates of employment in multiple sclerosis: A meta-analysis. J. Health Psychol. 2020, 25, 92–104.
- Dutta, R.; Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007, 68 (Suppl. 3), S22–S31.
- Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682.
- Dąbrowski, G.; Skrajda, M. Cannabinoids from Cannabis sp.: Mechanism of their activity and potential health benefits in human body. J. Educ. Health Sport 2017, 7, 936–945.
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 2012, 13, 23–34.
- Li, H.L. An Archaeological and Historical Account of Cannabis in China on JSTOR. Econ. Bot. 1974, 28, 437–448.
- Messam, C.A.; Hou, J.; Janabi, N.; Monaco, M.C.; Gravell, M.; Major, E.O. Glial Cell Types; Academic Press: New York, NY, USA, 2002; pp. 369–387.
- Kumar, M.R.P.; Vijayalakshmi, C.; Ramanathan, M. Isoflavones as Nutraceuticals in Stroke: Therapeutic Targets and Signaling Pathways. In Preedy Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2023; Chapter 50; pp. 959–978.
- Meyer, H.C.; Lee, F.S.; Gee, D.G. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology 2017, 43, 21–33.
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116.
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23.
- Pérez, J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today 2006, 42, 495–503.
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos. Trans. R Soc. B Biol. Sci. 2012, 367, 3254–3263.
- Gutiérrez-Rodríguez, A.; Bonilla-Del Río, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Robin, L.M.; et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia 2018, 66, 1417–1431.
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gomez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose metabolism links astroglial mitochondria to cannabinoid effects. Nature 2020, 583, 603–608.
- Tucci, V. (Ed.) Handbook of Neurobehavioral Genetics and Phenotyping; John Wiley & Sons: Hoboken, NJ, USA, 2017; Available online: https://books.google.com.eg/books?hl=en&lr=&id=n7RpDgAAQBAJ&oi=fnd&pg=PA25&ots=P4OtI2xJyg&sig=tfTwPhLxsI_9J7uUHVj3bwiZqvM&redir_esc=y#v=onepage&q&f=false (accessed on 3 July 2022).
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; Van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88.
- Kister, I.; Bacon, T.E.; Chamot, E.; Salter, A.R.; Cutter, G.R.; Kalina, J.T.; Herbert, J. Natural History of Multiple Sclerosis Symptoms. Int. J. MS Care 2013, 15, 146–156.
- Wegener, N.; Koch, M. Neurobiology and systems physiology of the endocannabinoid system. Pharmacopsychiatry 2009, 42 (Suppl. S1), S79–S86.
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445.
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74.
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141.
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Δ-9-Tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-Dependent Pathway. J. Immunol. 2000, 165, 373–380.
- Klein, T.W.; Kawakami, Y.; Newton, C.; Friedman, H. Marijuana components suppress induction and cytolytic function of murine cytotoxic T cells in vitro and in vivo. J. Toxicol. Environ. Health Part A Curr. Issues 2009, 32, 465–477.
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid. Res. 2020, 5, 12–31.
- Berdyshev, E.V.; Boichot, E.; Germain, N.; Allain, N.; Anger, J.P.; Lagente, V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur. J. Pharmacol. 1997, 330, 231–240.
- Sánchez, C.; Velasco, G.; Guzmán, M. Metabolic stimulation of mouse spleen lymphocytes by low doses of 9-tetrahydrocannabinol. Life Sci. 1997, 60, 1709–1717.
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory potential of cannabidiol in multiple sclerosis: A systematic review. J. Neuroimmune Pharmacol. 2021, 16, 251–269.
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782.
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519.
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456.
- Bunman, S.; Muengtaweepongsa, S.; Piyayotai, D.; Charlermroj, R.; Kanjana, K.; Kaew-Amdee, S.; Makornwattana, M.; Kim, S. Analgesic and Anti-Inflammatory Effects of 1% Topical Cannabidiol Gel in Animal Models. Cannabis Cannabinoid Res. 2023; Ahead of Print.
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The anti-inflammatory and analgesic effects of formulated full-spectrum cannabis extract in the treatment of neuropathic pain associated with multiple sclerosis. Inflamm. Res. 2020, 69, 549–558.
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 16, 346–362.
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol, ameliorates experimental multiple sclerosis by suppressing neuroinflammation through regulation of miRNA-mediated signaling pathways. Front. Immunol. 2019, 10, 1921.
- Nahas, G.G.; Morishima, A.; Desoize, B. Effects of cannabinoids on macromolecular synthesis and replication of cultured lymphocytes. Fed. Proc. 1977, 36, 1748–1752. Available online: https://europepmc.org/article/med/844617 (accessed on 13 June 2022).
- Derocq, J.M.; Ségui, M.; Marchand, J.; le Fur, G.; Casellas, P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995, 369, 177–182.
- Yang, X.; Bam, M.; Nagarkatti, S.; Nagarkatti, M. Cannabidiol Regulates Gene Expression in Encephalitogenic T cells Using Histone Methylation and noncoding RNA during Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2019, 9, 15780.
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the Th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276.
- González-García, C.; Torres, I.M.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; López, A.J.S. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp. Neurol. 2017, 298, 57–67.
- Klein, T.W.; Newton, C.; Friedman, H. Cannabinoid receptors and immunity. Immunol. Today 1998, 19, 373–381.
- Ojha, S.; Kumar, B. A review on nanotechnology based innovations in diagnosis and treatment of multiple sclerosis. J. Cell Immunother. 2018, 4, 56–64.
- Zeng, Y.; Li, Z.; Zhu, H.; Gu, Z.; Zhang, H.; Luo, K. Recent Advances in Nanomedicines for Multiple Sclerosis Therapy. ACS Appl. Bio. Mater. 2020, 3, 6571–6597.
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7.
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360.
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469.
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796.
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840.
- Goldsmith, M.; Abramovitz, L.; Peer, D. Precision Nanomedicine in Neurodegenerative Diseases. ACS Nano 2014, 8, 1958–1965.
- Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205.
- De Rosa, G.; Salzano, G.; Caraglia, M.; Abbruzzese, A. Nanotechnologies: A strategy to overcome blood-brain barrier. Curr. Drug Metab. 2012, 13, 61–69.
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. eLife 2017, 6, e25281.
- Zhang, C.; Feng, W.; Vodovozova, E.; Tretiakova, D.; Boldyrevd, I.; Li, Y.; Kürths, J.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Photodynamic opening of the blood-brain barrier to high weight molecules and liposomes through an optical clearing skull window. Biomed. Opt. Express 2018, 9, 4850–4862.
- Aryal, M.; Arvanitis, C.D.; Alexander, M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109.
- Song, K.-H.; Harvey, B.K.; Borden, M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 2018, 8, 4393.
- Hwang, D.; Dismuke, T.; Tikunov, A.; Rosen, E.P.; Kagel, J.R.; Ramsey, J.D.; Lim, C.; Zamboni, W.; Kabanov, A.V.; Gershon, T.R. Poly (2-oxazoline) nanoparticle delivery enhances the therapeutic potential of vismodegib for medulloblastoma by improving CNS pharmacokinetics and reducing systemic toxicity. Nanomedicine 2021, 32, 102345.
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575.
- Tylawsky, D.E.; Kiguchi, H.; Vaynshteyn, J.; Gerwin, J.; Shah, J.; Islam, T.; Boyer, J.A.; Boué, D.R.; Snuderl, M.; Greenblatt, M.B.; et al. P-selectin-targeted nanocarriers induce active crossing of the blood–brain barrier via caveolin-1-dependent transcytosis. Nat. Mater. 2023, 22, 391–399.
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, blood–brain barrier, and brain disposition. Pharmaceutics 2020, 12, 265.
- Adusumilli, N.C.; Hazuka, E.L.; Friedman, A.J. Nanotechnology to deliver cannabinoids in dermatology. Precis. Nanomed. 2021, 4, 787–794.
- Gajofatto, A.; Benedetti, M.D. Treatment strategies for multiple sclerosis: When to start, when to change, when to stop? World J. Clin. Cases WJCC 2015, 3, 545.
- Lucas, C.J.; Galettis; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477.
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219.
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360.
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.I. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188.
- Onaivi, E.S.; Chauhan, B.P.S.; Sharma, V. Challenges of cannabinoid delivery: How can nanomedicine help? Nanomedicine 2020, 15, 2023–2028.
- Rebelatto, E.R.L.; Rauber, G.S.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727.
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid nanocapsules decorated and loaded with cannabidiol as targeted prolonged release carriers for glioma therapy: In vitro screening of critical parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137.
- Durán-Lobato, M.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Receptor-targeted nanoparticles modulate cannabinoid anticancer activity through delayed cell internalization. Sci. Rep. 2022, 12, 1–17.
- Bruni, N.; della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478.
- Stella, B.; Baratta, F.; della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513.
More
