HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase inhibitors, commonly known as statins, are the primary treatment choice for cardiovascular diseases, which stand as the leading global cause of mortality. Statins also offer various pleiotropic effects, including improved endothelial function, anti-inflammatory properties, reduced oxidative stress, anti-thrombotic effects, and the stabilization of atherosclerotic plaques. However, the usage of statins can be accompanied by a range of adverse effects, such as the development of type 2 diabetes mellitus, muscular symptoms, liver toxicity, kidney diseases, cataracts, hemorrhagic strokes, and psychiatric complications. These issues are referred to as statin-associated symptoms (SAS) and are relatively infrequent in clinical trials, making it challenging to attribute them to statin use definitively. Therefore, these symptoms can lead to significant problems, necessitating dose adjustments or discontinuation of statin therapy.
- statin
- pleiotropic effects
- statin-associated symptoms
1. Introduction
2. Pleiotropic Benefits of Statin
2.1. Enhancing Endothelial Function
The onset of atherosclerosis often involves endothelial dysfunction, which is influenced by established cardiovascular risk factors such as hypertension, smoking, and high blood sugar levels. These factors, mediated by nitric oxide (NO), can interfere with the regular vasodilation process. Statins play a role in inhibiting the prenylation of Rac and Rho proteins, leading to an increase in the production of endothelium-derived nitric oxide synthetase (eNOS). This, in turn, enhances the production of nitric oxide and supports vasodilation [23][36]. This increased eNOS expression results in the elevated production of nitric oxide within the endothelium, which supports vasodilation [24][25][37,38]. NO release serves not only to facilitate vasodilation but also to hinder leukocyte adhesion, prevent platelet aggregation, and reduce the proliferation of vascular smooth muscle. As a result, NO plays a protective role, and inadequate levels indicate a greater risk of cardiovascular events [26][39]. A schematic representation in Figure 13 shows alterations in the vessel wall and endothelial cell membrane that occur during hyperlipidemia. These changes include LDL introduction, monocyte adhesion, platelet aggregation, foam cell formation, and the accumulation of cholesterol crystals in the intima. Alterations in the endothelial cell membrane include the presence of cholesterol-rich domains and increased caveolae, where caveolin plays a key role in regulating eNOS. These modifications are attenuated by HMG-CoA reductase inhibitors, which lower LDL levels and inhibit cholesterol and isoprenoid biosynthesis pathways [25][38].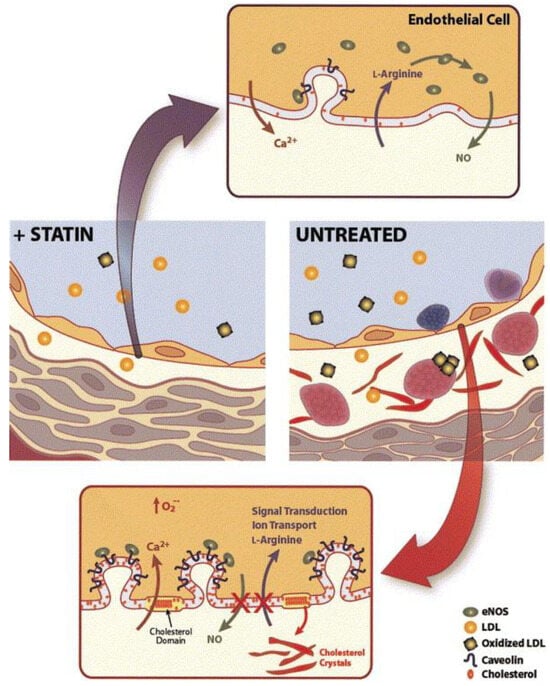
2.2. Plaque Stabilization
2.2. Plaque Stabilization
2.3. Anti-Inflammatory Effects
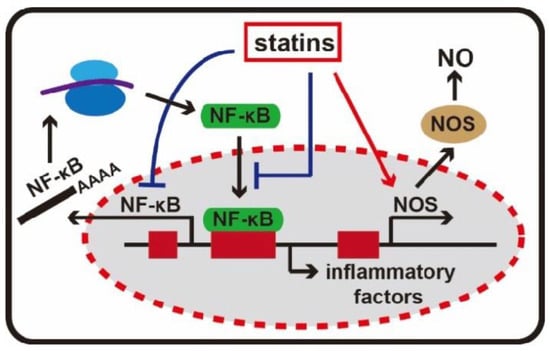
After endothelial damage, atherosclerotic plaques undergo infiltration of inflammatory cells. Statins can mitigate this inflammation by efficiently decreasing the production of inflammatory indicators, including C-reactive protein (CRP), serum amyloid A (SAA), interleukins, and adhesion molecules such as intracellular adhesion molecules (ICAM-I). These markers have all been associated with the initiation and recurrence of cardiovascular events. As illustrated in2.4. Immunomodulatory Effects
There is informal evidence to suggest that statins may possess anti-inflammatory and immunomodulatory properties, which could be beneficial in conditions such as cardiac transplant rejection and various autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, lupus, vasculitis, and systemic sclerosis [51]. Statins inhibit the induction of MHC-II expression by interferon γ (IFN-γ), leading to the repression of MHC-II-mediated T-cell activation. This effect is due to the inhibition of the inducible promoter IV of the transactivator CIITA and is observed in various cell types. MHC-II molecules play a crucial role in antigen presentation and T-cell activation through the T-cell receptor (TCR). TCR activation can impact T-cell proliferation, differentiation, and cytokine release. Cytokines released by activated T cells stimulate further T-cell proliferation, activate antigen-presenting cells (APCs), and promote B-cell antibody production. CD4+ helper T cells (TH cells) can differentiate into two distinct effector cell populations, TH1 and TH2, each producing different cytokines. Shifting the balance from TH1 to TH2 responses is beneficial in diseases characterized by delayed-type hypersensitivity reactions, such as graft atherosclerosis and chronic inflammatory conditions. Statins can induce this shift from TH1 to TH2 lymphocytes [52,53].2.4. Immunomodulatory Effects
2.5. Anti-Thrombotic Effects
2.5. Anti-Thrombotic Effects
2.6. Reduced Oxidative Stress
Many studies suggest that statins have the potential to reduce oxidative stress through various mechanisms, including the inhibition of ROS production, enhancement of antioxidant defenses, protection of endothelial cells, and anti-inflammatory effects [46][60]. The metabolites of atorvastatin, particularly hydroxy metabolites, exhibit the ability to inhibit the oxidation of LDL, HDL, and VLDL particles. This suggests that statins may exert an antioxidant effect that could contribute to impeding the progression of atherosclerosis independently of their LDL-lowering effects [47][61]. Statins not only hinder the production of cholesterol but also disrupt the generation of Rac1, a protein involved in activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the production of reactive oxygen species (ROS) [48][62]. These ROS can contribute to various negative effects such as endothelial dysfunction, inflammation, and oxidation of LDL particles, all of which play roles in the development of atherosclerosis [49][63].2.7. Protection from High-Decibel Noise-Inducing Hearing Loss
In a recent study, researchers evaluated the potential of statins as a treatment for hearing loss in CBA/CaJ mice [50][64]. They investigated the effects of delivering fluvastatin directly to the cochlea and administering lovastatin orally, assessing hearing outcomes using methods such as auditory brain stem responses (ABRs). Mice were exposed to two hours of octave band noise. Previous research with guinea pigs had demonstrated the protective effects of fluvastatin in the contralateral cochlea. Exposure to high-intensity noise (120 dB SPL for 4 h at 4–8 kHz) was found to result in the loss of hair cells—a phenomenon absent in guinea pigs subjected to noise but treated with fluvastatin. [51][65].2.8. Enhance Responses to Immune Checkpoint Blockade in Cancer Models
In the early stages of statin research, concerns arose about a potential link between statin use and cancer risk, particularly with lipophilic statins such as simvastatin [52][66]. Early observational studies suggested a link between statins and an increased risk of certain cancers [53][67]. This concern originated from the understanding that statins, acting as cholesterol-lowering agents, influence cellular processes associated with cancer development. The reduction in cholesterol, crucial for cell membrane integrity, raised theoretical concerns about long-term consequences. However, as more robust studies unfolded, these early apprehensions lacked consistent support.3. Adverse Effects of Statin Therapy
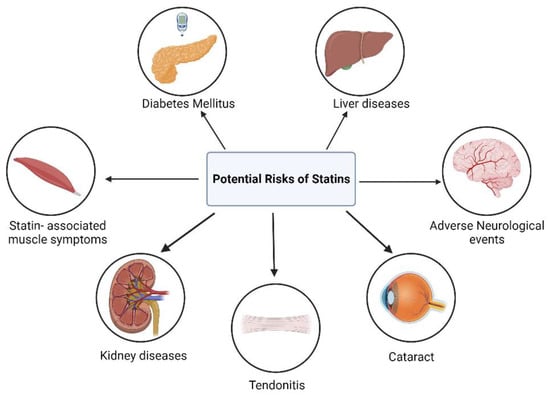
Statin therapy has frequently been associated with several unintended adverse effects, which further contribute to the concept of statin pleiotropy (Figure 36).