Constant technological development of modern imaging has led to substantial improvement in management and decision-making in the diagnostic and prognostic process of many different neoplasms. This also applies to cervical cancer. The main evidence, providing the base of recently updated ESGO-ESTRO-ESP recommendations (2023) on the management and treatment of cervical cancer, has been evaluated and reviewed in this paper. Ultrasound has been suggested as a valid alternative to MRI in primary diagnostic workup of cervical cancer if performed by an expert sonographer. Additionally, CT or PET/CT exhibits a substantial role in assessing the extrapelvic spread of the disease in locally advanced cases or when suspicious lymph nodes are detected.
- cervical cancer
- staging
- ultrasound
- MRI
- CT
- PET-CT
- neoplasm
- diagnostic imaging
1. Introduction
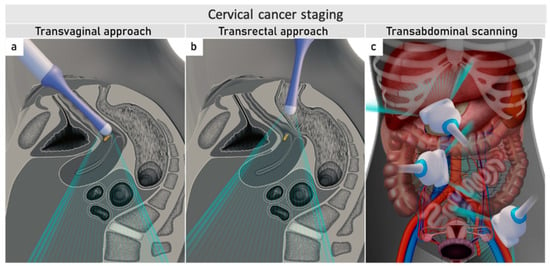
2. Local (Pelvic) Workup for Different Stages
The role of modern imaging in local staging is to delineate the cervical tumour to determine if fertility-sparing surgery can be offered, tailor the radicality of parametrial resection based on the minimum thickness of uninvolved cervical stroma, and to assess parametrial infiltration and tumour invasion into the pelvic side wall or adjacent organs (bladder, rectum). Following the revised FIGO 2018 staging system for cervical cancer [11][12], imaging methods include ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), combined PET-CT or PET-MRI, etc., based on local resources [13]. In the ESGO survey published in 2018, CT, PET-CT, MRI, and ultrasound were frequently used in the pre-treatment diagnostic workup. The survey showed that in early-stage disease, MRI was the most frequently used imaging method (74%), but more than half of the respondents used CT (54%), and a minority preferred PET-CT (25%). Pelvic ultrasound was reportedly considered in 23% [8]. Real-life data on gynecologic oncologists’ preferred primary staging modality and their diagnostic performance in early-stage cervical cancer was published in the prospective, international SENTIX study [14]. Each participating site was instructed to choose their preferred method based on their routine clinical practice. Among 690 prospectively enrolled patients with early-stage cervical cancer, 46.7% and 43.2% of patients underwent MRI and pelvic ultrasound, respectively, whereas 10.1% underwent both modalities. Pelvic MRI and ultrasound yielded similar diagnostic performance for predicting histological tumour size, parametrial involvement, and macrometastatic nodal involvement. CT is a well-established imaging method being widely used in cancer staging. Improvements in CT technologies using helical and multi-detector CT (MDCT) yield higher spatial resolution with shorter acquisition times, albeit with reported slightly higher radiation exposure [15]. However, CT is still inferior to MRI in assessing tumour size and local tumour extension due to its lower soft-tissue contrast resolution, even when using contrast-enhanced (CE) CT [4][5][16][17][18]. Iodinated contrast agents are routinely used for CT examinations to visualise contrast-enhancing neoplastic lesions or metastases and diagnose various non-malignant conditions, e.g., infectious- and vascular disease (Figure 1) [4][16]. PET-CT provides a unique combination of anatomic information provided by CT and tissue-specific metabolic characteristics provided by PET using the glucose analogue (18)F-fluorodeoxyglucose (FDG). The fused images acquired during a single examination facilitate localising malignant lesions, typically exhibiting increased FDG-avidity and depiction of physiologic FDG uptake in non-malignant tissue. However, PET-CT is not optimal for local staging due to the low soft-tissue contrast on CT and the low spatial resolution of PET. Furthermore, the partial volume effect from FDG-avid urine in the bladder (physiologic renal FDG excretion) may preclude an accurate definition of the tumour volume or parametrial invasion (Figure 1) [19]. PET-CT involves slightly higher radiation exposure than diagnostic CT alone [20]. MRI thoroughly assesses the pelvic anatomy with a wide field of view and no radiation risk. MRI has for years been considered the modality of choice for detecting local tumour spread [21] and has been shown to yield high interobserver reproducibility for tumour size measurements with high concordance between maximum primary tumour size from MRI and from hysterectomy specimens [22]. However, MRI is relatively expensive and time-consuming and may be contraindicated in some patients (e.g., in the presence of MRI-incompatible implants). Furthermore, limitations in access to MRI scanners are particularly common in low-income countries. As for all imaging modalities, their diagnostic accuracy depends on the radiologist’s experience in gynaecologic oncologic imaging. The MRI protocol, traditionally based on morphological sequences, has recently been supplemented by functional sequences, including diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI (Figure 1) [23][24]. DWI depicts the free water motion of the tissue. The free water movement is restricted in malignant lesions, normally being highly cellular. The tumour appears hyperintense on high b-value (e.g., b = 1000 s/mm2) images and correspondingly hypointense on the apparent diffusion coefficient (ADC) maps. The ADC map depicts true restricted diffusion and allows measurements of tumour ADC value. DWI combined with conventional MRI sequences enable assessments of both morphologic and physiologic features in a single examination. In DCE-MRI, dynamic image acquisition is accomplished after the administration of an intravenous bolus of gadolinium-based contrast agent. Typically, cervical tumours enhance rapidly, followed by an early washout of contrast. In the early arterial phase (30 sec post-contrast), the tumour is hyperintense. In contrast, in the late venous phase (2 min post-contrast), it is hypointense relative to the more gradually enhancing normal cervical epithelium and stroma [17][23]. Using a contrast agent could increase the reader’s confidence in identifying stromal and parametrial invasion. On the other hand, no significant improvement in staging accuracy has been demonstrated, and therefore, its use is not considered essential [25]. The addition of DWI to T2-weighted MRI demonstrated a promising role in improving the detection of parametrial invasion and increasing reader confidence [26][27], allowing better tumour delineation for less-experienced radiologists. Importantly, the measured maximum tumour dimensions are reportedly virtually identical based on DWI and conventional series. Functional magnetic resonance, including DWI and DCE-MRI imaging, have also been recently addressed and studied as a tool supplementing conventional MRI in brachytherapy settings for patients with locally advanced cervical cancer. Their complementary use resulted in lower interobserver variability in target delineation (Gross tumour volume) [28]. Nevertheless, validation through robust prospective data, before extensive adoption of DWI and DCE-MRI in cervical cancer, is essential. Particular attention must be paid to the use of uniform protocols, standardised nomenclature and correlation of imaging findings with histopathology [29][30]. PET-MRI integration has not yet been shown to significantly improve local staging performance compared to MRI alone [31]. Examination from the renal hila to the pubic symphysis is recommended to assess the presence of hydronephrosis in case of pelvic wall and lymph node infiltration (see below) [24].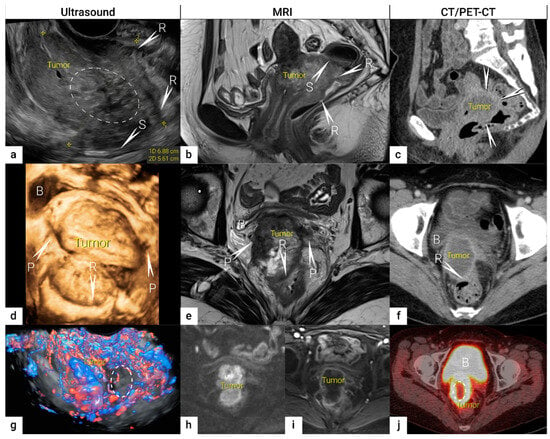
|
Parameters |
Expert Ultrasound |
MRI |
CECT |
FDG-PET-CT |
|---|---|---|---|---|
|
Cost |
1× |
4× |
2× |
6× |
|
Availability |
Specialised centers |
Most hospitals |
Most hospitals |
Specialised centers |
|
Range of examination |
Abdomen and pelvis, peripheral lymph nodes |
Whole body |
Whole body |
Whole body |
|
Examination duration (minutes) |
15–30 # |
30–45 (pelvic) 60 (whole-body) ~15 (reading time) |
5 ~10 (reading time) |
30 ~20 (reading time) |
|
Dynamic evaluation * |
Yes |
No |
No |
No |
|
Preparation before imaging |
None |
Antiperistaltic agents |
None |
4 h fasting and 1 h rest prior to scanning |
|
Contrast agent |
None ** |
Gadolinium-based |
Iodine-based |
FDG-radiotracer and iodine-based |
|
Radiation exposure |
None |
None |
10–20 mSv ß |
20–25 mSv ß |
|
Limitations for application and factors impacting diagnostic quality |
No contraindications. Limited depiction of abdominal deeper structures when overlying bowel gas/air |
Contraindication if severe claustrophobia, and for some metal- or cochlear implants/cardiac pacemakers. Image artefacts from implants. |
Contraindication for iodine-based contrast agent: - renal insufficiency & - hyperthyroidism - severe iodine allergy Image artefacts from implants. |
Contraindication for iodine-based contrast agent: - renal insufficiency & - hyperthyroidism - severe iodine allergy Image artefacts from implants. |
|
Dependence of expertise |
Yes |
Yes |
Yes |
Yes |
2.1. Tumour Detection
The first step in local staging by ultrasound or MRI is the identification of cervical cancer tissue, which is assessed in relation to the surrounding cervical stroma. On ultrasound, a squamous cell cancer is characterised by a hypoechogenic, richly vascularised tumour, while adenocarcinoma is more often an iso- or hyperechogenic, highly perfused lesion contrasting with healthy residual cervical stroma (Figure 4) [50]. On T2-weighted MRI, cervical cancer is typically an iso- or hyperintense mass regardless of histopathologic type, and when small, is surrounded by a hypointense normal cervical stroma (Figure 4) [51]. Tumour hyperintensity on the high b-value DWI with corresponding low intensity on the ADC map represents true restricted diffusion in the lesion. Importantly, the inclusion of DWI in addition to T2-weighted imaging in cervical cancer has been shown to improve tumour detection and reader confidence and yield better diagnostic accuracies for predicting parametrial invasion [27][52]. Promising results on early-stage tumour detection by ultrasound versus MRI based on single-unit studies [34][35] have been reported by a European multicentre trial including 189 patients operated for early-stage cervical cancer [36]. The transvaginal or transrectal ultrasound/MRI (based on T2-weighted and T1-weighted series without DWI) yielded accuracies (sensitivities) [specificities] of 96%/86%, p < 0.001 (90%/67%, p = 0.008) [97%/89%, p = 0.005] for tumour detection. This research found an excellent agreement between ultrasound and final histology for detecting tumours (kappa value 0.84), while the agreement between MRI and final histology was only moderate (0.52).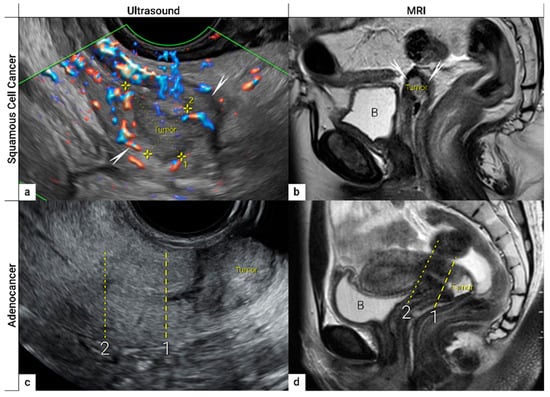
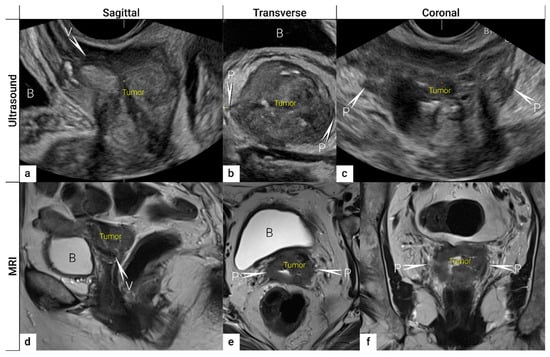
2.2. Tumour Delineation within Cervix (Tumour Size, Depth of Stromal Invasion, Minimum of Uninvolved Stroma, and Cranial Tumour-Free Margin)
The next step is to delineate tumour borders within the cervix to assess important prognostic parameters (tumour size, depth of stromal invasion, minimum of uninvolved stroma, and cranial tumour-free margin and others), which guide the treatment options and enable individualised management, including fertility-sparing treatment (FST). To assess eligibility for FST, the joint ESGO-ESTRO-ESP cervical cancer guidelines recommend ultrasound and MRI as the imaging tests of choice to measure remaining cervical length (after cone biopsy), uninvolved cranial tumour-free margin, and residual tumour size (Figure 4) [9]. FST is only feasible in squamous cell cancer or HPV-associated adenocarcinoma with a largest diameter of less than or equal to 2 cm [9]. A multicentric European trial focused on the tumour detection rate after cone biopsy, comparing the diagnostic performance of ultrasound and MRI (without DWI) [36]. The residual tumour detection rate after cone biopsy was not significantly different from tumour detection rate after cervical biopsy alone if assessed on ultrasound (sensitivity: p = 1.0; specificity: p = 0.23) or MRI (sensitivity: p = 0.78; specificity: p = 0.25) [36]. The study reported good agreement between ultrasound and MRI in classifying small tumours less than 2 cm (kappa values 0.78 and 0.71, respectively). For FST planning, the most important parameter to be evaluated is the actual tumour-free distance between the cranial tumour margin and the internal os (Figure 4). A recent meta-analysis published by Xiao et al. in 2020 on the diagnostic performance of MRI in evaluating the distance between the tumour and the internal os on six studies (454 patients) showed a pooled sensitivity and specificity of 87 and 91%, respectively [53]. A meta-analysis published by Woo et al. in 2020 analysed five MRI studies and showed a pooled sensitivity of 84% and specificity of 96% in detecting internal os involvement [54]. A multi-institutional prospective study compared the accuracy of ultrasound and MRI for tumour detection, tumour size measurements, parametrial-, uterine corpus- and vaginal fornix involvement and prediction of FIGO/T-status (from TNM system), reporting no significant difference between the two imaging methods [55]. Regarding assessment of cervical internal os invasion and uterine corpus infiltration as a negative prognostic factor, the accuracy for ultrasound and MRI was 94% and 86%, respectively (p = 0.3) [55]. On top of that, ultrasound can also be used to guide the surgeon intraoperatively during FST to determine the optimal level of excision in order to preserve the maximum length of tumour-free cervix for a future pregnancy [56]. In the pilot study, the cranial tumour-free margin was marked intraoperatively under ultrasound guidance with a non-absorbable suture [56]. The pathology report showed a mean distance between the stitch and the cranial tumour margin of 1.5 mm (SD 1.16, range 0.09–5 mm) [56]. In cases in which neoadjuvant chemotherapy (NACT) precedes FST with the aim to achieve partial or complete tumour response with sufficient cranial tumour-free margin, MRI or ultrasound in experienced hands can also be used to assess the treatment response [57]. In a single-unit prospective study comparing MRI and ultrasound after NACT in 42 patients using pathological results as a reference, the agreement between measurements obtained by MRI (without DWI) and histology was not found statistically significant (intraclass correlation coefficient; 0.344; 95% CI: −0.013 to 0.610; p = 0.059), while agreement between transrectal ultrasound and histology reached statistical significance (intraclass correlation coefficient; 0.795; 95% CI: 0.569–0.902; p < 0.001) [57]. Individualised planning of care is not only limited to FST but also to assess the radicality of hysterectomy or primary chemoradiotherapy, depending on the tumour size and depth of stromal invasion. Tumour size >4 cm and deep stromal invasion are indicative of a worse prognosis; thus, they are used in many centres as an indication for primary chemoradiation instead of primary surgery. The multicentric European trial results demonstrated almost perfect agreement between ultrasound and histology in the assessment of such bulky tumours (>4 cm) and deep stromal invasion (kappa values 0.82 and 0.81, respectively) [36]. The agreement between MRI (without DWI) and histology was substantial for the classification of bulky tumours (>4 cm) and detection of deep stromal invasion (kappa values 0.76 and 0.77, respectively) [36]. Recently, the measurement of the distance between tumour and parametria (tumour-free distance or a minimum uninvolved stroma) has been recommended as it better correlates with the risk of extrauterine extension and nodal metastasis rate than the tumour size or depth of stromal invasion, which does not consider the size of the cervix and the tumour location within the cervix [58][59]. It is measured as the remaining uninvolved fibromuscular stroma between the tumour and pericervical fascia at the point where the ventral, lateral, and dorsal parametria are attached to the cervix. The cut-off values for tumour-free distance associated with clinical outcomes ranged between 2.5 and 3.5 mm but without prospective validation [58][59]. Looking at the available evidence, pre-surgical MRI showed a sensitivity of 88% and a specificity of 75% in the assessment of tumour-free distance [60].2.3. Extrauterine Extension (Vagina, Parametria, Pelvic Side Wall, Hydronephrosis and Others)
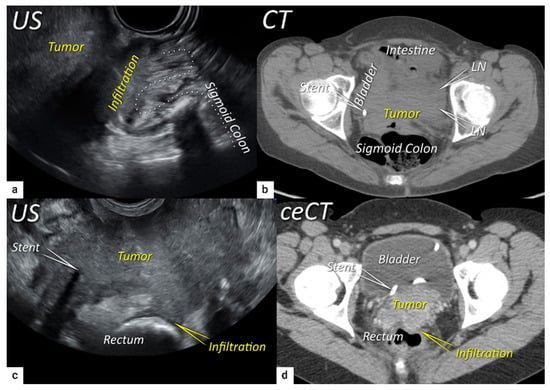
The third step is the assessment of extrauterine extension. The vaginal extension is routinely assessed during physical examination. The estimation of vaginal fornix using imaging can be difficult especially in large tumours stretching the vaginal fornix. An optional, useful tool to better assess the vaginal extension of the tumours is represented by vaginal opacification with gel, especially when the region of interest is represented by the posterior vaginal fornix. On the other hand, the role of imaging is critical to assess pericervical fascia and parametrial involvement, including pelvic side wall invasion. Visualisation of intact pericervical fascia surrounding the cervix as a hyperechogenic line on ultrasound or a hypointense line on MRI excludes infiltration of parametria with specificity and a negative predictive value of 98–100% [34][61][62]. In addition, the dynamic aspect of ultrasound examination helps to establish parametrial status. Especially in situations with limited visibility, the exertion of sliding of the tumour against the surrounding tissue planes is a sign of intact parametria. A multicentric European trial of early-stage cervical cancer of transvaginal or transrectal ultrasound/MRI (based on T2-weighted and T1-weighted series without DWI) yielded accuracies (sensitivities) [specificities] of 97%/90%, p = 0.001 (77%/69%, p = 0.56) [98%/92%, p < 0.001] in the assessment of parametrial invasion [36]. A recent meta-analysis published by Alcázar et al. in 2020 reported similar diagnostic performance for detecting parametrial invasion in cervical cancer by ultrasound/MRI, with pooled sensitivities and specificities of 78%/68% and 96%/91%, respectively [63]. No statistical differences were found when comparing both methods (p = 0.548) [63]. These data were confirmed by another meta-analysis published in 2020 by Woo et al. and showed that ultrasound has a comparable level of diagnostic performance to MRI in assessing parametrial invasion (pooled sensitivities and specificities of 67%/71% and 94%/91%, respectively) [54]. Apart from establishing the involvement of parametria, the precise localisation of infiltration (ventral right/left, lateral right/left, dorsal right/left) represents an added benefit for radiotherapy planning (Figure 1 and Figure 3) [32]. A narrated video by Moro et al. guided the reader through the methodology of assessing the structures surrounding the cervix and vagina (specifically the parametrium) [64]. Chiappa et al. compared the agreement between 2D and 3D ultrasound to MRI results as a reference in assessing parametrial invasion and showed the best agreement in the assessment of ventral parametria (90% and 62.5%), followed by the right lateral parametrium (72% and 81%), left lateral parametrium (69% and 70%), and dorsal parametria (58.5% and 52%) [32]. Based on the study results, 2D- and 3D-ultrasound showed similar moderate agreement with MRI. In addition to the location of infiltrated parametria, the degree of parametrial invasion can also be assessed on ultrasound or MRI using a standardised grading system, which is crucial for the treatment choice [33]. The features of parametrial invasion are incipient infiltration of pericervical fascia (usually in depth ≤5 mm), grade 2; nodular infiltration of parametrium, grade 3, discontinual parametrial involvement (“skip-metastasis”), grade 4 [33]. Metastatic visceral paracervical lymph nodes are considered discontinual parametrial invasion on ultrasound (grade 4) [34]. TNM and FIGO do not define how to classify metastases in the para-uterine visceral lymph nodes [12][65]. Since the lymphatic spread from cervical cancer is initially to these visceral (parametrial) lymph nodes drained by internal iliac vessels (e.g., uterine vessels), their involvement could be classified as locoregional lymph node metastases and not as infiltrated parametria. Parametrial invasion towards the pelvic side wall upstages the disease from T2b to T3b [12][65]. The pelvic side wall is defined as the parietal muscles of the lesser pelvis (obturator internus, coccygeus, and piriformis muscle), fascia, neurovascular structures, or skeletal portions of the bony pelvis. Pelvic side wall invasion by a tumour is a frequent cause of ureteric obstruction associated with hydronephrosis (Figure 5). Additional use of transabdominal ultrasound is essential for screening of hydronephrosis with a sensitivity of 76.5%, specificity and positive predictive value of 100%, negative predictive value of 85%, and accuracy of 90% [43]. The location of ureteric obstruction can be easily identified using a combination of transabdominal and transvaginal/transrectal ultrasound or MRI following the visualisation of suprastenotic dilatation of the ureter. Hydronephrosis on MRI can be examined if the protocol includes a sequence with an extended field of view to both kidneys in the axial or coronal plane. The degree of hydronephrosis is divided into three grades, as has been previously described [33].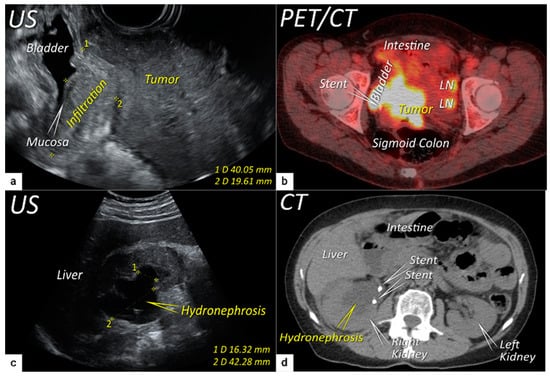
2.4. Extension to Surrounding Organs (Bladder, Rectum, Sigmoid Colon)
The last step in local staging focuses on the assessment of tumour growth into the adjacent organs (bladder and rectum or sigmoid colon). The level of infiltration of both the bladder and rectum can be determined simultaneously using an identical grading system (see below) [33]. Ultrasound or MRI are used to detect the infiltration of the endopelvic fascia based on the assessment of the contact planes between adjacent organs and the extent of involvement of both the bladder and rectal wall (Figure 6 and Figure 7) [33][40].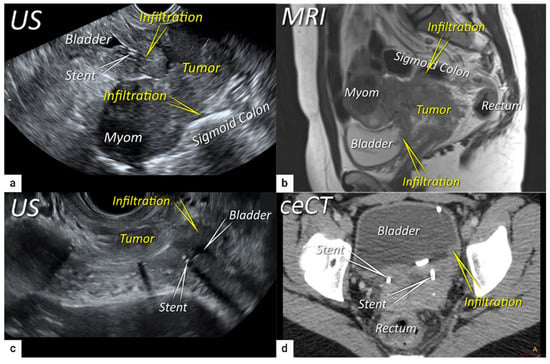
References
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S43–S103.
- Pecorelli, S.; Zigliani, L.; Odicino, F. Revised FIGO staging for carcinoma of the cervix. Int. J. Gynecol. Obstet. 2009, 105, 107–108.
- Innocenti, P.; Pulli, F.; Savino, L.; Nicolucci, A.; Pandimiglio, A.; Menchi, I.; Massi, G. Staging of cervical cancer: Reliability of transrectal US. Radiology 1992, 185, 201–205.
- Mitchell, D.G.; Snyder, B.; Coakley, F.; Reinhold, C.; Thomas, G.; Amendola, M.; Schwartz, L.H.; Woodward, P.; Pannu, H.; Hricak, H. Early Invasive Cervical Cancer: Tumor Delineation by Magnetic Resonance Imaging, Computed Tomography, and Clinical Examination, Verified by Pathologic Results, in the ACRIN 6651/GOG 183 Intergroup Study. J. Clin. Oncol. 2006, 24, 5687–5694.
- Hricak, H.; Gatsonis, C.; Chi, D.S.; Amendola, M.A.; Brandt, K.; Schwartz, L.H.; Koelliker, S.; Siegelman, E.S.; Brown, J.J.; McGhee, R.B.; et al. Role of Imaging in Pretreatment Evaluation of Early Invasive Cervical Cancer: Results of the Intergroup Study American College of Radiology Imaging Network 6651–Gynecologic Oncology Group 183. J. Clin. Oncol. 2005, 23, 9329–9337.
- Thomeer, M.G.; Gerestein, C.; Spronk, S.; van Doorn, H.C.; van der Ham, E.; Hunink, M.G. Clinical examination versus magnetic resonance imaging in the pretreatment staging of cervical carcinoma: Systematic review and meta-analysis. Eur. Radiol. 2013, 23, 2005–2018.
- Amendola, M.A.; Hricak, H.; Mitchell, D.G.; Snyder, B.; Chi, D.S.; Long, H.J., 3rd; Fiorica, J.V.; Gatsonis, C. Utilization of diagnostic studies in the pretreatment evaluation of invasive cervical cancer in the United States: Results of intergroup protocol ACRIN 6651/GOG 183. J. Clin. Oncol. 2005, 23, 7454–7459.
- Dostalek, L.; Åvall-Lundqvist, E.; Creutzberg, C.L.; Kurdiani, D.; Ponce, J.; Dostalkova, I.; Cibula, D. ESGO Survey on Current Practice in the Management of Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 1226–1231.
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655.
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666.
- Bhatla, N.; Denny, L. FIGO Cancer Report 2018. Int. J. Gynecol. Obstet. 2018, 143, 2–3.
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135.
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 22–36.
- Cibula, D.J.J.; Kocian, R.; Dundr, P.; Klat, J.; Zapardiel, I.; Landoni, L.; van Lonkhuijzen, L.; Frühauf, F.; Zikan, M.; Siegler, K.; et al. Magnetic resonance or expert ultrasound in preoperative local staging of patients with early-stage cervical cancer: Final results of the SENTIX prospective, single-arm, international trial (CEEGOG CX-01; ENGOT-CX2). Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2023, 33, A2.
- A Tsalafoutas, I.; Koukourakis, G.V. Patient dose considerations in computed tomography examinations. World J. Radiol. 2010, 2, 262–268.
- Bipat, S.; Glas, A.S.; van der Velden, J.; Zwinderman, A.H.; Bossuyt, P.M.; Stoker, J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: A systematic review. Gynecol. Oncol. 2003, 91, 59–66.
- Haldorsen, I.S.; Lura, N.; Blaakær, J.; Fischerova, D.; Werner, H.M.J. What Is the Role of Imaging at Primary Diagnostic Work-Up in Uterine Cervical Cancer? Curr. Oncol. Rep. 2019, 21, 77.
- Lee, S.I.; Catalano, O.A.; Dehdashti, F. Evaluation of Gynecologic Cancer with MR Imaging, 18F-FDG PET/CT, and PET/MR Imaging. J. Nucl. Med. 2015, 56, 436–443.
- Lazzari, R.; Cecconi, A.; Jereczek-Fossa, B.A.; Travaini, L.L.; Dell’ Acqua, V.; Cattani, F.; Rizzo, S.; Fodor, C.; Landoni, F.; Orecchia, R. The role of FDG-PET/CT in staging and treatment planning for volumetric modulated Rapidarc radiotherapy in cervical cancer: Experience of the European Institute of Oncology, Milan, Italy. Ecancermedicalscience 2014, 8, 405.
- Dwarakanath, B.S.; Kaushik, A.; Jaimini, A.; Tripathi, M.; D′Souza, M.; Sharma, R.; Mondal, A.; Mishra, A.K. Estimation of radiation dose to patients from FDG whole body PET/CT investigations using dynamic PET scan protocol. Indian J. Med. Res. 2015, 142, 721–730.
- Balleyguier, C.; Sala, E.; Da Cunha, T.; Bergman, A.; Brkljacic, B.; Danza, F.; Forstner, R.; Hamm, B.; Kubik-Huch, R.; Lopez, C.; et al. Staging of uterine cervical cancer with MRI: Guidelines of the European Society of Urogenital Radiology. Eur. Radiol. 2010, 21, 1102–1110.
- Lura, N.; Wagner-Larsen, K.S.; Forsse, D.; Trovik, J.; Halle, M.K.; Bertelsen, B.I.; Salvesen, Ø.; Woie, K.; Krakstad, C.; Haldorsen, I.S. What MRI-based tumor size measurement is best for predicting long-term survival in uterine cervical cancer? Insights Imaging 2022, 13, 105.
- Lucas, R.; Dias, J.L.; Cunha, T.M. Added value of diffusion-weighted MRI in detection of cervical cancer recurrence: Comparison with morphologic and dynamic contrast-enhanced MRI sequences. Diagn. Interv. Radiol. 2015, 21, 368–375.
- Manganaro, L.; Lakhman, Y.; Bharwani, N.; Gui, B.; Gigli, S.; Vinci, V.; Rizzo, S.; Kido, A.; Cunha, T.M.; Sala, E.; et al. Staging, recurrence and follow-up of uterine cervical cancer using MRI: Updated Guidelines of the European Society of Urogenital Radiology after revised FIGO staging 2018. Eur. Radiol. 2021, 31, 7802–7816.
- Sala, E.; Rockall, A.; Rangarajan, D.; Kubik-Huch, R.A. The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur. J. Radiol. 2010, 76, 367–385.
- Park, J.J.; Kim, C.K.; Park, S.Y.; Park, B.K.; Kim, B. Value of diffusion-weighted imaging in predicting parametrial invasion in stage IA2–IIA cervical cancer. Eur. Radiol. 2014, 24, 1081–1088.
- Qu, J.-R.; Qin, L.; Li, X.; Luo, J.-P.; Li, J.; Zhang, H.-K.; Wang, L.; Shao, N.-N.; Zhang, S.-N.; Li, Y.-L.; et al. Predicting Parametrial Invasion in Cervical Carcinoma (Stages IB1, IB2, and IIA): Diagnostic Accuracy of T2-Weighted Imaging Combined With DWI at 3 T. Am. J. Roentgenol. 2018, 210, 677–684.
- Han, K.; Croke, J.; Foltz, W.; Metser, U.; Xie, J.; Shek, T.; Driscoll, B.; Ménard, C.; Vines, D.; Coolens, C.; et al. A prospective study of DWI, DCE-MRI and FDG PET imaging for target delineation in brachytherapy for cervical cancer. Radiother. Oncol. 2016, 120, 519–525.
- Chopra, S.; Kundu, S.; Verma, A.; Mahantshetty, U.; Engineer, R.; Shrivastava, S.K. Functional magnetic resonance imaging in cervical cancer: Current evidence and future directions. J. Cancer Res. Ther. 2012, 8, 11–18.
- Matani, H.; Patel, A.K.; Horne, Z.D.; Beriwal, S. Utilization of functional MRI in the diagnosis and management of cervical cancer. Front. Oncol. 2022, 12, 1030967.
- Sarabhai, T.; Schaarschmidt, B.M.; Wetter, A.; Kirchner, J.; Aktas, B.; Forsting, M.; Ruhlmann, V.; Herrmann, K.; Umutlu, L.; Grueneisen, J. Comparison of 18F-FDG PET/MRI and MRI for pre-therapeutic tumor staging of patients with primary cancer of the uterine cervix. Eur. J. Nucl. Med. 2017, 45, 67–76.
- Chiappa, V.; Di Legge, A.; Valentini, A.L.; Gui, B.; Miccò, M.; Ludovisi, M.; Giansiracusa, C.; Testa, A.C.; Valentin, L. Agreement of two-dimensional and three-dimensional transvaginal ultrasound with magnetic resonance imaging in assessment of parametrial infiltration in cervical cancer. Ultrasound Obstet. Gynecol. 2015, 45, 459–469.
- Fischerova, D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumors: A review. Ultrasound Obstet. Gynecol. 2011, 38, 246–266.
- Fischerova, D.; Cibula, D.; Stenhova, H.; Vondrichova, H.; Calda, P.; Zikan, M.; Freitag, P.; Slama, J.; Dundr, P.; Belacek, J. Transrectal ultrasound and magnetic resonance imaging in staging of early cervical cancer. Int. J. Gynecol. Cancer 2008, 18, 766–772.
- Testa, A.C.; Ludovisi, M.; Manfredi, R.; Zannoni, G.; Gui, B.; Basso, D.; Di Legge, A.; Licameli, A.; Di Bidino, R.; Scambia, G.; et al. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound Obstet. Gynecol. 2009, 34, 335–344.
- Epstein, E.; Testa, A.; Gaurilcikas, A.; Di Legge, A.; Ameye, L.; Atstupenaite, V.; Valentini, A.L.; Gui, B.; Wallengren, N.-O.; Pudaric, S.; et al. Early-stage cervical cancer: Tumor delineation by magnetic resonance imaging and ultrasound—A European multicenter trial. Gynecol. Oncol. 2013, 128, 449–453.
- Szabó, G.; Madár, I.; Hudelist, G.; Arányi, Z.; Turtóczki, K.; Rigó, J.; Ács, N.; Lipták, L.; Fancsovits, V.; Bokor, A. Visualization of sacral nerve roots and sacral plexus on gynecological transvaginal ultrasound: Feasibility study. Ultrasound Obstet. Gynecol. 2023, 62, 290–299.
- Fischerova, D.; Santos, G.; Wong, L.; Yulzari, V.; Bennett, R.J.; Dundr, P.; Burgetova, A.; Barsa, P.; Szabó, G.; Sousa, N.; et al. Imaging in gynecological disease (26): Clinical and ultrasound characteristics of benign retroperitoneal pelvic peripheral-nerve-sheath tumors. Ultrasound Obstet. Gynecol. 2023, 62, 727–738.
- Iwamoto, K.; Kigawa, J.; Minagawa, Y.; Miura, H.; Terakawa, N. Transvaginal ultrasonographic diagnosis of bladder-wall invasion in patients with cervical cancer. Obstet. Gynecol. 1994, 83, 217–219.
- Huang, W.; Yang, J.; Yang, Y.; Yang, S. Ultrasonographic characteristics and cystoscopic correlates of bladder wall invasion by endophytic cervical cancer. Ultrasound Obstet. Gynecol. 2006, 27, 680–686.
- Testa, A.C.; Ferrandina, G.; Moro, F.; Pasciuto, T.; Moruzzi, M.C.; De Blasis, I.; Mascilini, F.; Foti, E.; Autorino, R.; Collarino, A.; et al. PRospective Imaging of CErvical cancer and neoadjuvant treatment (PRICE) study: Role of ultrasound to predict partial response in locally advanced cervical cancer patients undergoing chemoradiation and radical surgery. Ultrasound Obstet. Gynecol. 2018, 51, 684–695.
- Alcázar, J.L.; Castillo, G.; Martínez-Monge, R.; Jurado, M. Transvaginal color Doppler sonography for predicting response to concurrent chemoradiotherapy for locally advanced cervical carcinoma. J. Clin. Ultrasound 2004, 32, 267–272.
- Vanderpuye, V. Renal sonography in the diagnosis of renal obstruction or hydronephrosis in patients with cervical cancer. J. Clin. Ultrasound 2002, 30, 424–427.
- Pálsdóttir, K.; Epstein, E. A Pilot Study on Diagnostic Performance of Contrast-Enhanced Ultrasonography for Detection of Early Cervical Cancer. Ultrasound Med. Biol. 2018, 44, 1664–1671.
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2018, 39, e2–e44.
- Hricak, H.; Gatsonis, C.; Coakley, F.V.; Snyder, B.; Reinhold, C.; Schwartz, L.H.; Woodward, P.J.; Pannu, H.K.; Amendola, M.; Mitchell, D.G. Early Invasive Cervical Cancer: CT and MR Imaging in Preoperative Evaluation—ACRIN/GOG Comparative Study of Diagnostic Performance and Interobserver Variability. Radiology 2007, 245, 491–498.
- Pálsdóttir, K.; Fridsten, S.; Blomqvist, L.; Alagic, Z.; Fischerova, D.; Gaurilcikas, A.; Hasselrot, K.; Jäderling, F.; Testa, A.C.; Sundin, A.; et al. Interobserver agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound Obstet. Gynecol. 2021, 58, 773–779.
- Federico, M.; Hernandez-Socorro, C.R.; Ribeiro, I.; Martin, J.G.; Oramas, M.D.R.-B.; Saez-Bravo, M.L.; Jimenez, P.C.L. Prospective intra/inter-observer evaluation of pre-brachytherapy cervical cancer tumor width measured in TRUS and MR imaging. Radiat. Oncol. 2019, 14, 173.
- Wagner-Larsen, K.S.; Lura, N.; Salvesen, O.; Salvesen Halle, M.K.; Forsse, D.; Trovik, J.; Smit, N.; Krakstad, C.; Haldorsen, I.S. Interobserver agreement and prognostic impact for MRI–based 2018 FIGO staging parameters in uterine cervical cancer. Eur. Radiol. 2022, 32, 6444–6455.
- Epstein, E.; Di Legge, A.; MÅsbäck, A.; Lindqvist, P.G.; Kannisto, P.; Testa, A.C. Sonographic characteristics of squamous cell cancer and adenocarcinoma of the uterine cervix. Ultrasound Obstet. Gynecol. 2010, 36, 512–516.
- Okamoto, Y.; Tanaka, Y.O.; Nishida, M.; Tsunoda, H.; Yoshikawa, H.; Itai, Y. MR Imaging of the Uterine Cervix: Imaging-Pathologic Correlation. RadioGraphics 2003, 23, 425–445.
- Vandecaveye, V.; Dresen, R.; De Keyzer, F. Novel imaging techniques in gynaecological cancer. Curr. Opin. Oncol. 2017, 29, 335–342.
- Xiao, M.; Yan, B.; Li, Y.; Lu, J.; Qiang, J. Diagnostic performance of MR imaging in evaluating prognostic factors in patients with cervical cancer: A meta-analysis. Eur. Radiol. 2019, 30, 1405–1418.
- Woo, S.; Atun, R.; Ward, Z.J.; Scott, A.M.; Hricak, H.; Vargas, H.A. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: Systematic review and meta-analysis. Eur. Radiol. 2020, 30, 5560–5577.
- Stukan, M.; Buderath, P.; Szulczyński, B.; Gębicki, J.; Kimmig, R. Accuracy of Ultrasonography and Magnetic Resonance Imaging for Preoperative Staging of Cervical Cancer—Analysis of Patients from the Prospective Study on Total Mesometrial Resection. Diagnostics 2021, 11, 1749.
- Pinkavova, I.; Dundr, P.; Fischerova, D.; Zikan, M.; Slama, J.; Cibula, D. OC24.04: Intra-operative ultrasound in fertility sparing procedures for cervical cancer. Ultrasound Obstet. Gynecol. 2012, 40, 51.
- Pinkavova, I.; Fischerova, D.; Zikan, M.; Burgetova, A.; Slama, J.; Svarovsky, J.; Dundr, P.; Dusek, L.; Cibula, D. Transrectal ultrasound and magnetic resonance imaging in the evaluation of tumor size following neoadjuvant chemotherapy for locally advanced cervical cancer. Ultrasound Obstet. Gynecol. 2013, 42, 705–712.
- Cibula, D.; Slama, J.; Dostálek, L.; Fischerová, D.; Germanova, A.; Frühauf, F.; Dundr, P.; Nemejcova, K.; Jarkovsky, J.; Sebestova, S.; et al. Tumour-free distance: A novel prognostic marker in patients with early-stage cervical cancer treated by primary surgery. Br. J. Cancer 2020, 124, 1121–1129.
- Bizzarri, N.; Anchora, L.P.; Zannoni, G.F.; Carbone, V.; Bruno, M.; Fedele, C.; Gallotta, V.; Chiantera, V.; Avesani, G.; Gui, B.; et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: A retrospective, single-centre, cohort study. Br. J. Cancer 2021, 125, 561–568.
- Rizzo, S.; Calareso, G.; Maccagnoni, S.; Angileri, S.A.; Landoni, F.; Raimondi, S.; Pasquali, E.; Lazzari, R.; Bellomi, M. Pre-operative MR evaluation of features that indicate the need of adjuvant therapies in early stage cervical cancer patients. A single-centre experience. Eur. J. Radiol. 2014, 83, 858–864.
- Kim, M.J.; Chung, J.J.; Lee, Y.H.; Lee, J.T.; Yoo, H.S. Comparison of the use of the transrectal surface coil and the pelvic phased-array coil in MR imaging for preoperative evaluation of uterine cervical carcinoma. Am. J. Roentgenol. 1997, 168, 1215–1221.
- Yu, K.K.; Hricak, H.; Subak, L.L.; Zaloudek, C.J.; Powell, C.B.; Yu, H.H.K.K.; Sala, E.; Wakely, S.; Senior, E.; Lomas, D.; et al. Preoperative staging of cervical carcinoma: Phased array coil fast spin-echo versus body coil spin-echo T2-weighted MR imaging. Am. J. Roentgenol. 1998, 171, 707–711.
- Alcazar, J.L.; García, E.; Machuca, M.; Quintana, R.; Escrig, J.; Chacón, E.; Mínguez, J.A.; Chiva, L. Magnetic resonance imaging and ultrasound for assessing parametrial infiltration in cervical cancer. A systematic review and meta-analysis. Med. Ultrason. 2020, 1, 85–93.
- Moro, F.; Zermano, S.; Ianieri, M.M.; Nardone, A.D.C.; Carfagna, P.; Ciccarone, F.; Ercoli, A.; Querleu, D.; Scambia, G.; Testa, A.C. Dynamic transvaginal ultrasound examination for assessing anatomy of parametrium. Ultrasound Obstet. Gynecol. 2023, 62, 904–906.
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J. Clin. 2021, 71, 287–298.
- Nicolet, V.; Carignan, L.; Bourdon, F.; Prosmanne, O. MR Imaging of Cervical Carcinoma: A Practical Staging Approach. RadioGraphics 2000, 20, 1539–1549.
- Rockall, A.G.; Ghosh, S.; Alexander-Sefre, F.; Babar, S.; Younis, M.T.S.; Naz, S.; Jacobs, I.J.; Reznek, R.H. Can MRI rule out bladder and rectal invasion in cervical cancer to help select patients for limited EUA? Gynecol. Oncol. 2006, 101, 244–249.
 Encyclopedia
Encyclopedia
