You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 3 by Camila Xu.
Apart from the isolation of single substances, complexly composed products, so-called multicomponent mixtures (more than one constituent substances, MOCSs), are obtained from the natural materials. A multicomponent mixture contains numerous individual constituents with different functionalities. By using the example of Lentinula edodes (Berk.) Pegler, shiitake, it is shown that this is the case not only for plants but also for medicinal mushrooms. Lentinan, a polysaccharide isolated from the mycelium, is used as a single compound as part of an integrated tumor therapy.
- Lentinula edodes
- shiitake
- medicinal mushrooms
- multicomponent mixtures
1. Introduction
In the history of mankind, plants and fungi were for a long time the only available means for the treatment of health problems. They were used in the form of whole plants and mushrooms or extracts prepared therefrom. Beginning in the 18th and 19th century, as individual constituents began to be increasingly isolated from plants and fungi, there has been increasing interest in the chemical composition of herbs and their constituents. A breakthrough was the extraction of morphine in 1806 by the pharmacist F.W. Sertürner (1783–1804) from opium (Papaver somniferum) [1]. This was quickly followed by other discoveries of active plant and fungi substances [2][3][4][5].
As a result, complex herbal and fungal preparations were increasingly replaced by isolated or synthesized pure substances. They were easier to dose and had often a stronger and better predictable effect than complex mixtures.
Nevertheless, plants, fungi, and, to a lesser degree, animals have retained their importance as biogenic drugs. In some regions of the world, they are still the only or nearly only source for medicinal treatment [6]. The Traditional Chinese Medicine (TCM) and other medicinal systems mainly from East Asia strongly rely on mixtures of several plants and/or fungi [7][8].
Apart from the isolation of single substances, complexly composed products, so-called multicomponent mixtures (more than one constituent substances, MOCSs), are obtained from the natural materials. A multicomponent mixture contains numerous individual constituents with different functionalities. Together, they contribute to the properties of the complete mixture. Typical MOCSs are extracts of herbal (including fungal) drugs, essential oils, or animal venoms [9][10].
2. Chemistry and Biological Effects of Single Compounds from Lentinula edodes
2.1. Overview
Table 1 gives an overview of the nutritionally relevant ingredients of Lentinula edodes, and Table 2 summarizes those substances that are primarily of pharmacological interest. However, the transitions between these two groups are fluid. For example, dietary fiber is a bulk-giving component of the diet that contributes significantly to eliminating the feeling of hunger. On the other hand, dietary fiber helps to promote peristalsis and maintain a healthy microbiome and can therefore also be regarded as a pharmacologically active ingredient. Another example is ergosterol, which itself is hardly pharmacologically active, but can be converted to the pharmacologically active ergocalciferol (vitamin D2).
Table 1. Composition of Lentinula edodes fruit bodies under special consideration of nutrients (according to [11] and references therein).
| Substance | Content | Remarks | |
|---|---|---|---|
| Dry matter | 8.4–20.2 g/100 g FW | ||
| Total dietary fiber | 32.4% of dry matter | high content of insoluble fiber including chitin | |
| Lentin, a 27.5 KDa protein | Antifungal; | Inhibition of HIV-1 reverse transcriptase; Inhibition of proliferation of leukemia cells |
[15] |
| Amino acids (additionally to proteinogenic amino acids) |
Ergothioneine | Antioxidative | [16] |
| γ-Amino butyric acid (GABA) | Neurotransmitter | [11] | |
| Nucleotide derivatives | Eritadenine (lentinacin, lentysin); Desoxyeritadenine. |
Antilipidemic; Inhibits angiotensin converting enzyme (ACE); Reduces homocysteine level |
[11][17] |
| 5’-Nucleotides | |||
| 2.42 g/100 g DW | |||
| Monophosphates of 5’-guanosine, 5’inosine and 5’xanthosine | |||
| 48% of 5’-Nucleotides | |||
| affect taste of fresh mushrooms | |||
DW: dry weight, FW: fresh weight.
Table 2. Components of Lentinula edodes with proved pharmacological activities and/or other special properties.
| Substance Group | Substance | Activity and/or other Properties | References | |||
|---|---|---|---|---|---|---|
| Polysaccharides | Lentinan | Immunomodulating | [12][13] | |||
| Proteins | Ledodin | Atypical ribosome-inactivating protein; Cytotoxic |
[14] | |||
| Ash | 4.3–6.7 g/100 g DW | |||||
| Energy | 33.2–77.2 kcal/100 g FW | |||||
| Crude protein | 4.4–21.4 g/100 g DW | |||||
| Essential amino acids | 32.0% of total amino acids | |||||
| [ | 18 | ] | [19] | Free amino acids | 1.25–3.16 g/100 g DW | |
| Cyclic-sulfur-containing compounds | 1,2,4-Trithiolan, arising from the non-volatile lentinic acid; 1,2,3,4,5,6-Hexathiocycloheptan (hexathiepan); | affect taste of fresh mushrooms | ||||
| 1,2,3,5,6-Pentathiepan (lentionin); 1,2,4,6-Tetrathiepan. |
Main odor substance | [11][12][20] | Crude fat | 0.9–6.3 g/100 g DW | ||
| Polyunsaturated fatty acids | ||||||
| Phenolics | 82.0% of total fatty acids | |||||
| p-Hydroxybenzoic acid; | Carbohydrates | 67.9–87.2 g/100 g DW | ||||
| Soluble sugars and polyols | Caps: 111.6 g/100 g DW Stipes: 125.0 g/100 g DW Dried powder: 38.3 g/100 g DW |
|||||
| Ergosterol | 217–495 mg/100 g DW | precursor of vitamin D2 | ||||
| Vanillic acid; | Syringic acid; p-Coumaric acid; Further Polyphenols. |
Antioxidative | [11][19][21][22] | Tocopherols | 5.4–32.3 µg/100 g DW | |
| Vitamin C | 25 mg/100 g DW | |||||
| Vitamin B1 | 0.6 mg/100 g DW | |||||
| Vitamin B2 | 1.8 mg/100 g DW | |||||
| Vitamin B12 | 0.8–5.6µg/100 g DW | |||||
| Folates | 0.3–0.66 mg/100 g DW | |||||
| Niacin/niacinamid | 31 mg/100 g DW |
In Table 2, content data are omitted since comparability is not given, due to different test materials and determination methods as well as often missing data in the literature.
Among the nutritionally important characteristics worth highlighting are the high content of dietary fiber and of proteins containing all essential amino acids, the presence of vitamins and minerals, and the low energy density. Also worth noting are the pleasant sensory properties for which the volatile compounds are mainly responsible [11][20][23][24][25][26][27].
2.2. Lentinan
The best studied biologically active substance is the polysaccharide lentinan (Figure 12). This glucan consists of β-1,3-linked glucose units to which β-1,6 side chains are attached after every five linear glucose residues. The secondary structure is probably a curdlan-type helix conformation, which can be easily converted into a triple helix by changing the external conditions. The average molecular mass is 5.2 to 6.9 × 104 Daltons. Lentinan can be isolated from hot water extracts of L. edodes by a sequence of fractional precipitation and chromatographic purification steps [12][13][28][29].
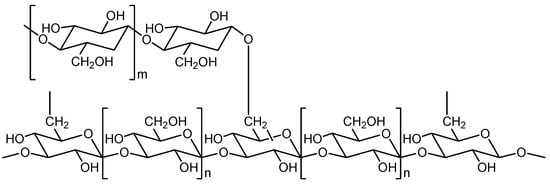
Figure 12. Partial structure of lentinan. n: the glucose unit in brackets is present n times in the 1,3-chain; m: the glucose unit in brackets is present m times in the 1,6-side chain.
Glucan molecules such as lentinan are considered to be pathogen-associated molecular patterns (PAMPs) targeting pattern recognition receptors (PRRs). They target dectin-1, complement receptor 3 (CR3), and further receptors on the surface of monocytes, macrophages, and other immune cells and are capable of stimulating both innate and adaptive immune responses (biological response modifier, BRM). Thus, numerous signaling pathways are activated. For example, this can lead to the release of cytokines, the strengthening of phagocytosis, and an oxidative burst response [30]. As a result, the host’s own immune defense against tumor cells can be stimulated. When applied parenterally (0.5–1 mg/day) or orally, lentinan can be used as part of an integrated tumor therapy together with chemotherapeutics and other measures (operation, radiation).
The immunostimulating effects of lentinan have been justified in numerous in vitro and in vivo investigations. For example, such studies show a stimulation of the release of various cytokines (IL-1α, IL-2, IFNγ, TNF α) and of the activity of natural killer cells (NK cells). In animal experiments, an inhibition of tumor growth could be achieved. Clinical studies and observations show an improvement in life quality and a prolongation of survival time of patients with mainly gastrointestinal tumors that had taken lentinan additionally to chemotherapy [23][30][31][32][33][34].
In the context of the CoV-19 pandemic, the antiviral activities of lentinan and other mushroom polysaccharides have gained increasing interest. The effects are mainly due to the immunostimulating properties of the compound [35]. β-Glucans derived from mycelia reduced cytokine levels involved in cytokine storms during CoV-19 infections and reduced lung infection [36]. Clinical studies of lentinan in HIV-positive patients showed promising results [37]. Antiviral effects against bovine herpes virus type 1, HIV, and HTLV-1 have also been shown for other polysaccharide preparations from L. edodes [23].
With regard to the other polysaccharides, it has to be mentioned that the extraction method and the extracted mushroom part (fruit bodies, mycelium, or spores) affect monosaccharide composition, molecular weight, degree of branching, and conformation and thus the biological activity of the compounds [30].
2.3. Eritadenine
Another well-studied constituent of L. edodes is the purine derivative eritadenine (Figure 23). The estimated content is between about 300 and 650 mg/100g DW. It decreases during cooking [17][18][19].
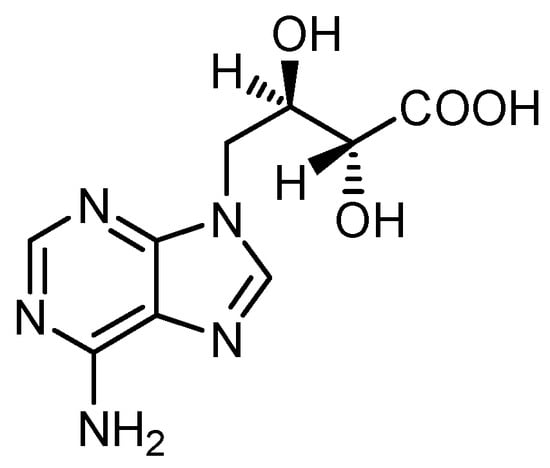
Figure 23. Structure of eritadenine.
In animal experiments, it lowers cholesterol and triglyceride levels by another mechanism than the well-known statins. Instead of the inhibition of HMG-CoA reductase as statins do, it modulates the expression of genes for enzymes of lipid metabolism and promotes the uptake of LDL into liver cells [38][39][40]. Additionally, eritadenine inhibits the activity of angiotensin-converting enzyme (ACE) in vitro and thus has possibly antihypertensive effects in vivo [18]. By the inhibition of S-adenosyl-L-homocysteine hydrolase, it reduces the high blood level of the amino acid homocysteine, a risk factor for cardiovascular and neurodegenerative diseases [40][41]. All these activities together can be expected to have positive effects in the prevention of atherosclerosis and other cardiovascular diseases.
Positive effects on lipid levels could also be achieved with isolated polysaccharides. In rats that were fed a lipid-rich diet, the polysaccharides reduced total cholesterol, triglycerides, and LDL cholesterol. The expression of the adhesion molecule VCAM-1mRNA in aortic endothelial cells that promotes the development of atherosclerotic plaques was decreased and the activity of antioxidative enzymes increased [42].
A third remarkable substance that should be mentioned is the amino acid ergothioneine (2-mercaptohistidine-trimethylbetain). Its content is strongly dependent on the culture conditions and estimated to be between 1.22 and 198 mg/100g DW. Ergothioneine has been proposed to be an adaptive antioxidant that may protect against the tissue damage implicated in several chronic diseases and promote healthy aging [16].
Among the other constituents, phenolics and volatiles (15 alcohols, 13 aldehydes, 9 alkanes, 5 sulfur-containing compounds, and other, [19]) should be mentioned.
Untargeted metabolomics of 7 mushroom species including L. edodes showed that 1344 compounds were detected in all 7 investigated species. A total of 472 compounds were unique to shiitake; 126 of them were annotated and 346 were not annotated [16]. This means that there is yet a vast unexplored field.
References
- Sertürner, F.A. Darstellung der reinen Mohnsäure (Opiumsäure) nebst einer Untersuchung des Opiums mit vorzüglicher Hinsicht auf einen darin neu entdeckten Stoff und die dahin gehörigen Bemerkungen. Vom Herrn Sertürner in Paderborn. J. Pharm. 1806, 14, 47–93.
- Barnes, J.; Anderson, L.A.; Phillipson, D. Herbal Medicines, 3rd ed.; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2007.
- Ferreira, T.S.; Moreira, C.Z.; Cária, N.Z.; Victoriano, G.; Silva, W.F., Jr.; Magalhães, J.C. Phytotherapy: An introduction to its history, use and application. Rev. Bras. Plantas Med. 2014, 18, 290–298.
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed. Pharmacol. 2018, 7, 1–7.
- Makarska-Białokoz, M. History and significance of phytotherapy in the human history. The development of phytotherapy from the Middle Ages to modern times. Arch. Physiother. Glob. Res. 2020, 24, 17–22.
- WHO. Traditional Medicine Strategy 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096. (accessed on 7 February 2024).
- Tang, W.; Eisenbrand, G. Handbook of Chinese Medicinal Plants: Chemistry, Pharmacology, Toxicology, 1st ed.; Wiley-VCH: Weinheim, Germany, 2011; Volumes I and II.
- Gongwang, L. Chinese Herbal Medicine, 1st ed.; Hua Xia Publishing House: Beijing, China, 2000.
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.; Schulz, H.; et al. Essential oils as examples for multicomponent mixtures–their potential for human health and well-being. Front. Pharmacol. 2022, 13, 956541.
- Wink, M. Current understanding of modes of action of multicomponent bioactive phytochemicals: Potential for nutraceuticals and antimicrobials. Ann. Rev. Food Sci. Technol. 2022, 13, 337–359.
- Kalač, P. Edible Mushrooms: Chemical Composition and Nutritional Value, 1st ed.; Academic Press: Cambridge, MA, USA, 2016.
- Lindequist, U. Lentinula edodes. In Hagers Handbuch der Pharmazeutischen Praxis; Schneider, G., Hänsel, R., Blaschek, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; Follow-up Volume 3, pp. 61–71.
- Chihara, G.; Maeda, Y.Y.; Suga, T.; Hamuro, J. Lentinan as a host defence potentiator (HDP). Int. J. Immunother. 1989, 5, 145–154.
- Citores, L.; Ragucci, S.; Russo, R.; Gay, C.C.; Chambery, A.; Di Maro, A.; Iglesias, R.; Ferreras, J.M. Structural and functional characterization of the cytotoxic protein ledodin, an atypical ribosome-inactivating protein from shiitake mushroom (Lentinula edodes). Protein Sci. 2023, 32, e4621.
- Ngai, P.H.; Ng, T.B. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003, 73, 3363–3374.
- Uffelman, C.N.; Doenges, K.A.; Armstrong, M.L.; Quinn, K.; Reisdorph, R.M.; Tang, M.; Krebs, N.F.; Reisdorph, N.A.; Campbell, W.W. Metabolomics profiling of white button, crimini, portabella, lion’s mane, maitake, oyster, and shittake mushrooms using untargeted metabolomics and targeted amino acid analysis. Foods 2023, 12, 2985.
- Enman, J.; Rova, U.; Berglund, K.A. Quantification of the bioactive compound eritadenine in selected strains of shiitake mushrooms (Lentinus edodes). J. Agric. Food Chem. 2007, 55, 1177–1180.
- Afrin, S.; Rakib, M.A.; Kim, B.H.; Kim, J.O.; Ha, Y.L. Eritadenine from edible mushrooms inhibits activity of angiotensin converting enzyme in vitro. J. Agric. Food Chem. 2016, 64, 2263–2268.
- Yao, F.; Gao, H.; Yin, C.M.; Shi, D.F.; Fan, X.Z. Effect of different cooking methods on the bioactive components, color, texture, microstructure, and volatiles of shiitake mushrooms. Foods 2023, 12, 2573.
- Hiraide, M.; Miyazaki, Y.; Shibata, Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: Relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004, 50, 358–364.
- Nam, M.; Choi, J.Y.; Kim, M.S. Metabolic profiles, bioactive compounds, and antioxidant capacity in Lentinula edodes cultivated on log versus sawdust substrates. Biomolecules 2021, 11, 1654.
- Choi, E.J.; Park, Z.Y.; Kim, E.K. Chemical composition and inhibitory effect of Lentinula edodes ethanolic extract on experimentally induced atopic dermatitis in vitro and in vivo. Molecules 2016, 21, 993.
- Finimundi, T.C.; Dillon, A.J.P.; Henriques, J.A.P.; Roesch Ely, M. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105.
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397.
- Cağlarirmak, N. The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds. Food Chem. 2007, 105, 1188–1194.
- Vetter, J. Mineralstoff- und Aminosäuregehalte des eßbaren, kultivierten Pilzes Shii-take (Lentinus edodes). Z. Lebensm. Unters. Forsch. 1995, 201, 17–19.
- Vetter, J. Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem. 2007, 102, 6–9.
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883.
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41.
- Roszczyk, A.; Turlo, J.; Zagożdżon; Kaleta, B. Immunomodulatory properties of polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980.
- Ochiai, T.; Isono, K.; Suzuki, T.; Koide, Y.; Gunji, Y.; Nagata, M.; Ogawa, N. Effect of immunotherapy with lentinan on patient’s survival and immunological parameters in patients with advanced gastric cancer: Results of a multi-centre randomized controlled study. Int. J. Immunother. 1992, VIII, 161–169.
- Vannucci, L.; Šima, P.; Vetvicka, V.; Křižan, J. Lentinan properties in anticancer therapy: A review on the last 12-year literature. Am. J. Immunol. 2017, 13, 50–61.
- Xiao, Z.; Jiang, Y.; Chen, X.F.; Wang, C.Q.; Zheng, X.T.; Xu, W.H.; Zou, X.X.; Zhou, J.M.; Yang, Y.H.; Hu, S.S.; et al. Intrathoracic infusion therapy with lentinan and chemical irritants for malignant pleural effusion: A systematic review and meta-analysis of 65 randomized clinical trials. Phytomedicine 2020, 76, 153260.
- Yin, X.; Ying, J.; Li, L.; Zhang, H.; Wang, H. A meta-analysis of lentinan injection combined with chemotherapy in the treatment of nonsmall cell lung cancer. Ind. J. Cancer 2015, 52, e29–e31.
- Baruah, P.; Patra, A.; Barge, S.; Khan, M.R.; Mukherjee, A.K. Therapeutic potential of bioactive compounds from edible mushrooms to attenuate SARS-CoV-2 infection and some complications of coronavirus disease (COVID-19). J. Fungi 2023, 9, 897.
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. ß-Glucans from the same edible shiitake mushroom Lentinus produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects–implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330.
- Gordon, M.; Bihari, B.; Goosby, E.; Gorter, R.; Greco, M.; Guralnik, M.; Mimura, T.; Rudinicki, V.; Wong, R.; Kaneko, Y. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: A phase I/II trial. J. Med. 1998, 29, 305–330.
- Chibata, L.; Okumura, K.; Takeyama, S.; Kotera, K. Lentinacin: A new hypocholesterolemic substance in Lentinus edodes. Experientia 1969, 25, 1237–1238.
- Shimada, Y.; Yamakawa, A.; Morita, T.; Sugiyama, K. Effects of dietary eritadenine on the liver microsomal Δ6-desaturase activity and its mRNA in rats. Biosci. Biotechnol. Biochem. 2003, 66, 1605–1609.
- Yamada, T.; Komoto, J.; Lou, K.; Ueki, A.; Hua, D.H.; Sugiyama, K.; Takata, Y.; Ogawa, H.; Takusagawa, F. Structure and function of eritadenine and its 3-deaza analogues: Potent inhibitors of S-adenosylhomocysteine hydrolase and hypocholesterolemic agents. Biochem. Pharmacol. 2007, 73, 981–989.
- Fukada, S.L.; Setoue, M.; Morita, T.; Sugiyma, K. Dietary eritadenine suppresses guanidinoacetic acid-induced hyperhomocysteinemia in rats. J. Nutr. 2006, 136, 2797–2802.
- Chen, X.; Zhong, H.Y.; Zeng, J.H.; Ge, J. The pharmacological effect of polysaccharides from Lentinus edodes on the oxidative status and expression of VCAM-1mRNA of thoracic aorta endothelial cell in high-fat-diet rats. Carbohydr. Polym. 2008, 74, 445–450.
More
