Polymer flooding is an enhanced oil recovery (EOR) method used to increase oil recovery from oil reservoirs beyond primary and secondary recovery. Although it is one of the most well-established methods of EOR, there are still continuous new developments and evaluations for this method. This is mainly attributed to the diverse polymers used, expansion of this method in terms of application, and the increase in knowledge pertaining to the topic due to the increase in laboratory testing and field applications.
- polymer injection
- polymer
- enhanced oil recovery (EOR)
1. Introduction
2. Polymer Flooding Principles and Mechanisms
Polymer flooding is considered a mobility control method. It can help to considerably improve areal and vertical sweep efficiencies if applied correctly; it can also help to increase the displacement efficiency. Mobility is defined as the ratio between a fluid’s permeability and its viscosity. As the mobility of a fluid increases, its ability to flow also increases. This corresponds to an increase in the fluid’s permeability and/or a decrease in its viscosity. Therefore, it is always preferable to increase the mobility of oil to increase the overall recovery [32][36]. When applying water flooding, as long as the mobility of the oil is higher than the mobility of the water, the oil flow behavior will be better; therefore, higher oil recovery will be observed. Since it is possible to calculate oil mobility, the same can be applied to water by dividing the water permeability by its viscosity. By comparing both the permeability of water and oil, it becomes clear which phase will flow more competitively. A common method to compare the two mobility values is the use of the mobility ratio. This is defined as the ratio between the mobility of the displacing phase and that of the displaced phase [30][32][33][34][35][36][37][38][33,34,35,36,37,38,39,40]. In all secondary and tertiary recovery applications, the displaced phase is the oil, whereas the displacing phase is the injected fluid that is being used to displace the oil. Based on this, it is always advisable to maintain a higher mobility of the displaced phase (oil) than that of the displacing phase (e.g., water or polymer). This is why the mobility ratio should be maintained at less than one for a favorable displacement process. Water can suffer from a premature increase in mobility ratio for two main reasons. First, it has a low viscosity value, which is comparable to or sometimes even less than that of oil. This can result in the water having a lower resistance to flow compared to oil, which, in turn, creates channels through the oil zone—a phenomenon referred to as viscous fingering [15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][32][33][34][35][36][37][38][39][18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. Secondly, depending on the water saturation and the wettability, the water can have a higher permeability than the oil. These two factors can result in a significant increase in the mobility ratio and, thus, a significant reduction in the overall oil recovery. The proposed solution to the premature increase in the mobility of the water is to target the two underlying causes of this increase. This can be achieved by significantly increasing the viscosity of the displacing phase (water). This also reduces the permeability of the water relative to the oil and creates a more uniform flood front. Therefore, a chemical is added to the water to increase its viscosity significantly beyond that of the oil; this is the fundamental concept of polymer flooding. By varying the type of polymer and its concentration, extremely high-viscosity values can be achieved [15][18]. This significantly decreases the mobility of the displacing phase and, thus, reduces the value of the mobility ratio below one for a longer duration. Based on this concept, polymer flooding is primarily classified as a mobility control method, since it maintains the mobility ratio below unity for as long as possible [3].3. Types of Polymers
Polymers are defined as long-chain chemicals composed of bonded, simply structured monomers. A polymer can be made of one monomer or multiple monomers, referred to as copolymers or a terpolymers [40][41][42][42,43,44]. The selection of the monomers depends on multiple factors, including:-
Temperature: The ability of the polymer to withstand high temperatures depends on an innate property of the polymer itself. This is a strong function of the main compounds that form the monomer; the type and orientation of the bonds present within the monomer structure; and, finally, the bond strength between the individual monomer units that form the long-chain polymer. The measure of the ability of the polymer to resist degradation under a specific temperature is referred to as thermal stability. Depending on the type of polymer, the temperature range for thermal stability can be extremely high according to previous research, ranging from 31 °C to 212 °C [43][45]. It is also important to note that for a specific polymer, multiple thermal stability ranges can be mentioned. The variation in the thermal stability values is the result of two main parameters. The first is the molecular weight of the polymer; this can vary significantly for the same polymer depending on the initiator used to link the monomers; the purity of the monomers; and the monomer density, which is reflected directly in the molecular weight, since the definition of molecular weight is the number of grams of the chemical per mole. The variation in thermal stability could also be due to variation in testing conditions [44][46]. Some researchers have focused on using freshwater and neutral pH when measuring thermal stability, whereas others have used tap water, which has a variety of salinity and pH values depending on the country and location. It is therefore highly advised to measure the thermal stability of each polymer individually before testing, since it may vary relative to the calculated value [45][47].
-
Pressure: Pressure is usually divided into two main types: injection pressure and the pressure differential during propagation into the formation. The ability of the polymer to withstand high pressure without failing is important to evaluate to avoid premature or excessive polymer degradation. It can be measured using pressure differential laboratory experiments involving core flooding [42][43][44][45][44,45,46,47].
-
pH: Different polymers can tolerate a wide range of pH values depending on the monomer used to develop the polymer. Although a wide range of polymers can function well under high pH values or basic conditions, a limited number of polymers can resist degradation under low pH values or acidic conditions. These polymers can be applied under highly acidic conditions such as in the presence of high concentrations of hydrogen sulfide or carbon dioxide or when the crude oil has a high total acid number. It is important to note that these types of polymers are usually much more costly than other conventional polymers [46][47][48][48,49,50].
-
Salinity: When referring to salinity, there are two main categories of interest: monovalent cations and divalent cations. Monovalent cations include any salt that is formed of an element with one valence electron, such as sodium, while divalent cations are salts that are formed of an element with two valence electrons, such as calcium. Salts have an overall negative impact on polymers, with monovalent cations being less damaging compared to divalent cations. A detailed description of the impact of salt in field applications of polymer flooding is presented in [30][33].
-
Rock Type: The type of rock involves both its lithology and its properties. The lithology of rock has an impact on the polymer if it can react with the polymer itself, causing polymer structural change and, eventually, degradation. The rock property that has the most significant impact on the polymer is wettability. If the polymer has a tendency to adhere to the rock surface, a large volume of the injected polymer is lost in the formation, failing to perform its function. This results in a significant increase in the polymer flooding cost [40][41][42][43][42,43,44,45].
-
Polymer Molecular Weight: The specific molecular weight of the polymer depends on the selected type of polymer and the method of polymerization. The molecular weight of the polymer impacts the concentration of polymer added to the water prior to injection, the injectivity of the polymer, and the ability of the polymer to propagate in different pore sizes in the formation. It is therefore extremely important to measure the molecular weight of the polymer prior to injection. It is also important to note that the molecular weight of the polymer can change over time due to polymer degradation [40][41][42][43][44][45][46][47][48][42,43,44,45,46,47,48,49,50].
-
Polymer Cost: The overall cost of a polymer flooding operation relies on multiple components, including the cost of the polymer itself. Depending on the polymer used, the cost can be extremely low, while for more specialized polymers, the cost of the polymer can be extremely high. The selection of the polymer itself is dependent on reservoir rock and fluid properties, reservoir thermodynamics, and operational considerations [47][49].
3.1. Organic Polymers
Examples of such polymers are xanthan gum (produced by the bacteria Xanthomonas campestris) and polysaccharides (obtained from sugar in a fermentation process caused by the bacterium Xanthomonas campestris). The molecular structure of polysaccharide biopolymers provides the molecules with considerable stiffness [49][51], so they behave like rigid-rod molecules [50][52]. Such a molecular structure and behavior allow the polysaccharide biopolymer to be salt-resistant; therefore, the viscosity of its solution is not affected by salinity. The main advantage of biopolymers compared to synthetic polymers is their environmental friendliness due to their organic nature. This type of polymer is not widely used to enhance oil recovery due to its high cost and its stability degradation at high temperatures (>200 °F). HPAM types of polymers are much more widely used than biopolymers, because HPAM has good water solubility and good surface activity, as well as stable rheological properties; it also offers advantages in terms of price and large-scale production, and its solutions exhibit significantly greater viscoelasticity than xanthan solutions. Polymers are used in aqueous solutions at low concentrations of 300 ppm (0.03%) to less than 2000 ppm (0.20%).3.2. Inorganic Polymers
An example of such a polymer is partially hydrolyzed polyacrylamide (HPAM), which is obtained by the polymerization reaction of acrylamide monomer [51][52][53][54][55][53,54,55,56,57]. Dissolving the obtained long molecular chain of HPAM in fresh water makes the fluid flow through tortuous porous media with ease. When the salinity of the water increases, the electrolytes in the polymeric solution cause the molecules to form a spiral shape that impedes the flow through the porous space and reduces the viscosity of the solution. Due to its sensitivity to salts, HPAM is usually injected between two slugs of fresh water, as shown in Figure 1. HPAM solutions are vulnerable to the presence of oxygen, high temperatures, and mechanical degradation. At and near the wellbore, high fluid velocities and temperatures may result in the breaking down of the long chains of HPAM molecules. HPAM is widely used for EOR due to its low cost and high resistance to being driven out of the formation [52][54].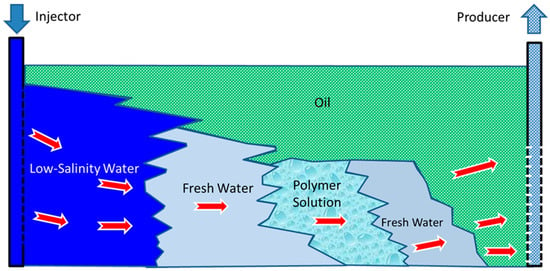
4. Polymer Flooding
Lab Scale
Many studies have investigated the use of polymers for different applications in laboratory-based experiments. These experiments investigated either the ability of a specific or a newly synthesized polymer to improve oil recovery or the impact of a specific parameter on polymer performance and degradation [32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49]. The majority of polymer flooding experiments conducted on polymer flooding EOR can be classified as core flooding experiments, polymer flooding in fractures, polymer application in microfluidics, or reservoir simulation of polymer flooding.
Core flooding is a broad term that refers to the injection of fluid into a confined core plug. The plug can be cylindrical or rectangular depending on the core holder. The dimensions of the plug can vary significantly depending on the core holder capacity and the type of experiment being conducted. Core flooding can include continuous cores or cores with fractures. Fractures involve two distinct modes: either continuous or partial fracture. Continuous fractures involve a fracture that covers the entire length of the core plug. A partial fracture is a fracture that propagates partially across the core [32][33][34].
Microfluidics studies the flow of the polymer through microchannels or porous media. The key difference between microfluidics and core flooding is the size of the observation, where microfluidics focuses on micro- and, sometimes, nanosized observations. Microfluidics is a key area of research with respect to the evaluation of the interactions occurring between polymers and rock, reservoir fluids, or both at the microscopic scale. Analysis of microchannels is usually conducted using imaging techniques such as magnifying microscopy, scanning electron microscopy, or transmission electron microscopy. There are several methods by which microfluidic channels can be created to model polymer flooding behavior in micropores. For all the methods summarized below, precision is key to producing a representative microfluidic rock sample for analysis [52][53][54][55][56][57][58][59][60][61].
Reservoir simulation of polymer flooding projects involves studying the ability of the polymer to increase oil recovery at the entire field scale rather than the small core scale. The polymer should be defined in the reservoir simulation process based on its properties and characteristics. This can be achieved by creating a new phase with unique properties, the most important of which is viscosity. It is important to note that many reservoir simulators do not take into consideration polymer degradation based on the change in conditions, assuming an ideal polymer [32][33][34][35][36][37][38][39].
Field Scale
Polymer dehydration is the process of the loss of water or fluid from the hydropolymer lattice. Loss of water can be due to several factors. An increase in temperature can result in significant and rapid polymer dehydration. The thermal stability of different polymers varies; therefore, dehydration should be tested to determine polymer compatibility with the reservoir. High pressure differentials can also result in polymer dehydration. This may be due to large depths, which require high injection pressure, or small pore sizes, which require high pressure for the polymer to propagate through the pores. Salinity is another significant factor that can impact polymer dehydration. When the formation water salinity is high compared to the hydropolymer water salinity, osmotic pressure causes the salt in the formation water to penetrate the polymer lattice. This results in polymer shrinkage and, therefore, loss of fluid. This can also result in polymer syneresis, which can damage the polymer structure and result in irreversible degradation [15][16][17][18][19][20][21][22][23][24].
Polymer degradation involves permanent damage to the polymer chains. Degradation can occur due to many factors depending on the polymer type and properties. Polymer degradation involves the weakening or destruction of the polymer chains, resulting in the polymer losing its high viscosity, which is attributed to the long chains and the high molecular weight of the polymer prior to degradation. Degradation is a strong function of the polymer limitations and can occur due to excessive temperature, pressure differentials, pH, salinity (including both monovalent and divalent cations), gasses such as carbon dioxide or hydrogen sulfide, pore size distribution in the formation, or shearing of the polymer during injection [57][58][59][60][61].
Polymer syneresis is the process of the loss of fluid from the polymer, which results in an increase in polymer rigidity and a significant reduction in polymer mobility. Syneresis differs from dehydration in that syneresis is usually associated with polymer structural degradation. This results in difficulty for the polymer structure in reabsorbing the lost fluid; therefore, the polymer degrades beyond return. In contrast, if the polymer dehydrates, it can be rehydrated by the addition of fluid, usually water. Polymer syneresis is impacted by the same factors that impact polymer degradation and dehydration.
During its injection, the polymer is subjected to extremely high shear rates. This shearing can damage the polymer chain and result in excessive polymer degradation. Some types of polymers can resist shearing more than others depending on their molecular structure. Vinyl acetate polymer can withstand some degree of shearing, for example, compared to weaker polymers such as acetic acid-based polymers. Although shearing cannot be entirely avoided, it can be reduced by proper design of the polymer injection on the surface. If the polymer is homogenously dissolved in the water, shearing tends to be significantly reduced. Decreasing the injection time and the injection pressure also tends to reduce polymer shearing [2][3][4][5][6][7][8][62][63].
5. Conclusions
4. Polymer Flooding
Lab Scale
Many studies have investigated the use of polymers for different applications in laboratory-based experiments. These experiments investigated either the ability of a specific or a newly synthesized polymer to improve oil recovery or the impact of a specific parameter on polymer performance and degradation [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The majority of polymer flooding experiments conducted on polymer flooding EOR can be classified as core flooding experiments, polymer flooding in fractures, polymer application in microfluidics, or reservoir simulation of polymer flooding.
Core flooding is a broad term that refers to the injection of fluid into a confined core plug. The plug can be cylindrical or rectangular depending on the core holder. The dimensions of the plug can vary significantly depending on the core holder capacity and the type of experiment being conducted. Core flooding can include continuous cores or cores with fractures. Fractures involve two distinct modes: either continuous or partial fracture. Continuous fractures involve a fracture that covers the entire length of the core plug. A partial fracture is a fracture that propagates partially across the core [34,35,36].
Microfluidics studies the flow of the polymer through microchannels or porous media. The key difference between microfluidics and core flooding is the size of the observation, where microfluidics focuses on micro- and, sometimes, nanosized observations. Microfluidics is a key area of research with respect to the evaluation of the interactions occurring between polymers and rock, reservoir fluids, or both at the microscopic scale. Analysis of microchannels is usually conducted using imaging techniques such as magnifying microscopy, scanning electron microscopy, or transmission electron microscopy. There are several methods by which microfluidic channels can be created to model polymer flooding behavior in micropores. For all the methods summarized below, precision is key to producing a representative microfluidic rock sample for analysis [54,55,56,57,58,59,60,61,62,63].
Reservoir simulation of polymer flooding projects involves studying the ability of the polymer to increase oil recovery at the entire field scale rather than the small core scale. The polymer should be defined in the reservoir simulation process based on its properties and characteristics. This can be achieved by creating a new phase with unique properties, the most important of which is viscosity. It is important to note that many reservoir simulators do not take into consideration polymer degradation based on the change in conditions, assuming an ideal polymer [34,35,36,37,38,39,40,41].
Field Scale
Polymer dehydration is the process of the loss of water or fluid from the hydropolymer lattice. Loss of water can be due to several factors. An increase in temperature can result in significant and rapid polymer dehydration. The thermal stability of different polymers varies; therefore, dehydration should be tested to determine polymer compatibility with the reservoir. High pressure differentials can also result in polymer dehydration. This may be due to large depths, which require high injection pressure, or small pore sizes, which require high pressure for the polymer to propagate through the pores. Salinity is another significant factor that can impact polymer dehydration. When the formation water salinity is high compared to the hydropolymer water salinity, osmotic pressure causes the salt in the formation water to penetrate the polymer lattice. This results in polymer shrinkage and, therefore, loss of fluid. This can also result in polymer syneresis, which can damage the polymer structure and result in irreversible degradation [18,19,20,21,22,23,24,25,26,27].
Polymer degradation involves permanent damage to the polymer chains. Degradation can occur due to many factors depending on the polymer type and properties. Polymer degradation involves the weakening or destruction of the polymer chains, resulting in the polymer losing its high viscosity, which is attributed to the long chains and the high molecular weight of the polymer prior to degradation. Degradation is a strong function of the polymer limitations and can occur due to excessive temperature, pressure differentials, pH, salinity (including both monovalent and divalent cations), gasses such as carbon dioxide or hydrogen sulfide, pore size distribution in the formation, or shearing of the polymer during injection [59,60,61,62,63].
Polymer syneresis is the process of the loss of fluid from the polymer, which results in an increase in polymer rigidity and a significant reduction in polymer mobility. Syneresis differs from dehydration in that syneresis is usually associated with polymer structural degradation. This results in difficulty for the polymer structure in reabsorbing the lost fluid; therefore, the polymer degrades beyond return. In contrast, if the polymer dehydrates, it can be rehydrated by the addition of fluid, usually water. Polymer syneresis is impacted by the same factors that impact polymer degradation and dehydration.
During its injection, the polymer is subjected to extremely high shear rates. This shearing can damage the polymer chain and result in excessive polymer degradation. Some types of polymers can resist shearing more than others depending on their molecular structure. Vinyl acetate polymer can withstand some degree of shearing, for example, compared to weaker polymers such as acetic acid-based polymers. Although shearing cannot be entirely avoided, it can be reduced by proper design of the polymer injection on the surface. If the polymer is homogenously dissolved in the water, shearing tends to be significantly reduced. Decreasing the injection time and the injection pressure also tends to reduce polymer shearing [2,3,4,5,6,7,8,9,10].
3. Conclusions
- Polymer-enhanced oil recovery is one of the most applied EOR methods in terms of both laboratory studies and field applications. The main factors impacting polymer injection are tied to polymer selection, reservoir rock and fluid properties, polymer concentration, and injection methodology.
- Polymer degradation is represented in multiple steps depending on the severity of impact. This can include the less severe dehydration and partial hydrolysis and the more severe shearing and syneresis.
- When coupled with other chemical EOR methods, polymers can be used in two main manners: either as a mechanism to support oil recovery by increasing sweep efficiency or as a sacrificial polymer to avoid the loss of the more costly surfactant to the formation.
- Some polymers can pose severe environmental concerns, especially if they react with underground fluids or break down into hazardous chemicals.
