Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract, which has become a serious threat to human health worldwide. CRC is a complex disease due to its extensive heterogeneity; thus, effective treatment could be enhanced by the implementation of a personalized medicine approach. Despite constantly improved diagnostic and individualized therapeutic methods, CRC remains one of the biggest problems of contemporary medicine. Knowledge of the basic risk factors, early clinical symptoms, and available screening tests, as well as the preservation of oncological alert, allow the proper targeting of the diagnostic process and, consequently, the earlier diagnosis of the disease. Undoubtedly, new research at the molecular and genetic level allows us to precisely understand the process of initiation and progression of cancerous diseases and, consequently, precise, personalized prevention and treatment.
- colorectal cancer
- epidemiology
- screening
- prophylaxis
- risk factors
- biomarkers
1. Introduction
2. The State of Knowledge from Symptoms to Novel Molecular Biology Achievements
2.1. Incidence of Colorectal Cancer and Its Geographic Variations
Throughout the world, CRC is nowadays a major cause of cancer-related morbidity and mortality, with nearly 1.9 million new cases diagnosed and almost 935,000 deaths in 2020 [1]. The global distribution of CRC has large geographic differences, with the number of new cases rapidly increasing due to population growth, changes in demographics, and the Westernization of lifestyle habits [6]. The highest incidence of CRC occurs in Europe, Australia/New Zealand, and North America [1]. CRC is both the third most commonly diagnosed cancer and the third most common cause of cancer-related deaths in both men and women in the United States [5]. In 2020, CRC was the most common type of cancer incidence among men in Slovakia, the countries of the Arabian Peninsula, Ethiopia, and Southeast Asian countries such as Singapore and Brunei Darussalam. It was also reported that CRC is the cancer with the highest mortality among men in countries of the Arabian Peninsula and Ethiopia and among women in Spain, Croatia, Belarus, Estonia, and Japan [1]. Two-thirds of CRC cases occur in countries characterized by high or very high indices of development and/or income. A pathological examination in approximately 95% of CRC cases reveals adenocarcinomas and other types of cancers that occur, including mucinous carcinomas and adenosquamous carcinomas [1,6][1][6].2.2. Temporal Trends in Colorectal Cancer Incidence, Mortality and Survival Rates
The incidence of CRC worldwide is constantly increasing, especially in countries characterized by a high-income economy and high human development index (HDI) [5,6,10][5][6][7]. CRC is considered one of the clearest markers of the cancer transition, replacing infection-related cancers in countries undergoing rapid social and economic changes. Such changes in the causes of cancer are predominantly linked to Western lifestyles and are, thus, frequently found in high-income countries. Given the temporal profiles and demographic projection, it is expected that by 2030, the global number of CRC cases will increase by 60% to more than 2.2 million new cases and approximately 1.1 million deaths annually [6]. Although over the past few decades, effective therapeutic strategies for CRC have been developed, the five-year overall survival is unsatisfactory. This is caused by the presence of the following poor prognostic factors: vascular, neural invasion, a low lymphocyte-to-monocyte ratio (LMR), late diagnosis, and tumor stage [12][8]. It is estimated that approximately 20% of CRC patients have already progressed into a metastatic state at the time of presentation, and more than 30% of patients with early CRC eventually develop metastatic disease [13,14][9][10]. CRC treatment’s long-term costs remain a huge social and economic burden [15][11].2.3. Symptoms of Colorectal Cancer
The course of CRC can be asymptomatic for a long time; the appearance of clinical symptoms is often indicated with an advanced stage of cancer. CRC symptomatology depends on the tumor localization. The classic symptoms of CRC include explicit or latent bleeding from the gastrointestinal tract (rectal bleeding or blood in the stool), abdominal pain, a palpably perceptible tumor, the rhythm of bowel movement disturbance (alternating constipation and diarrhea), gastrointestinal tract obstruction, unintended weight loss, fatigue, anemia, and febrile states [17,18][12][13]. Explicit bleeding from the lower digestive tract is common (in 50–60% of patients with CRC), and an easily observable symptom (per rectum examination) requires the determination of its cause in each case [19,20][14][15]. Another very common symptom is a bowel movement disorder (constipation/diarrhea), which occurs in about 50% of CRC cases; if it appears for more than 6 weeks without apparent cause, this becomes an alarming symptom [21][16]. An important symptom for CRC patients, which has been stated by various authors at a frequency of 30% to more than 75%, is anemia [18,22][13][17]. When diagnosing men over 40 years of age, as well as post-menopausal women with iron deficiency, such as anemia, the exclusion of a proliferative process is required. Weight loss, a palpable tumor, and abdominal pain are significantly associated with high levels of colorectal cancer.2.4. Pathological Evaluation of Colorectal Carcinogenesis
The WHO pathologic classification divides CRC into the following histologic subtypes: glandular cancers (classic adenocarcinomas, AC, the highest percentage of diagnoses), mucinous adenocarcinomas (MAC), signet cell carcinomas (SRCC), and variants including adenosquamous carcinomas, squamous cell carcinomas, medullary carcinomas, neuroendocrine, undifferentiated and others [24][18]. CRC is a cancer of epithelial origin, usually developing based on conventional adenomas. In total, 70% of all CRCs stem from adenomas. Progressions from adenoma to carcinoma take more than 10 years in sporadic cancer, whereas much shorter intervals can be observed in the Lynch syndrome [25][19]. Adenomas are distributed relatively throughout the colon; those with a flat or depressed morphology are distributed more in the proximal colon, and pedunculated lesions are more in the distal colon. Adenomas are, by definition, dysplastic, with the majority being low-grade; they can be characterized by tubular or villous histology, with the overwhelming majority being tubular. The increasing size of adenomas is associated with villous elements and invasive cancer (invasive cancer in adenomas ≤5 mm is extremely rare). An “advanced” stage is defined as a lesion ≥1 cm in size or having high-grade dysplasia or villous elements [26][20].2.5. Epigenetics of CRC
Evidence from scientific reports suggests an important role of epigenetic modifications in the development of CRC (Figure 21) [18,28,29,32,33,34,35][13][21][22][23][24][25][26]. Epigenetic modifications consist of changes in the methylation of cytosine-guanine (CpG) dinucleotides (DNA methylation), histone-tail post-translational modifications, and the expression of non-coding RNAs (ncRNA).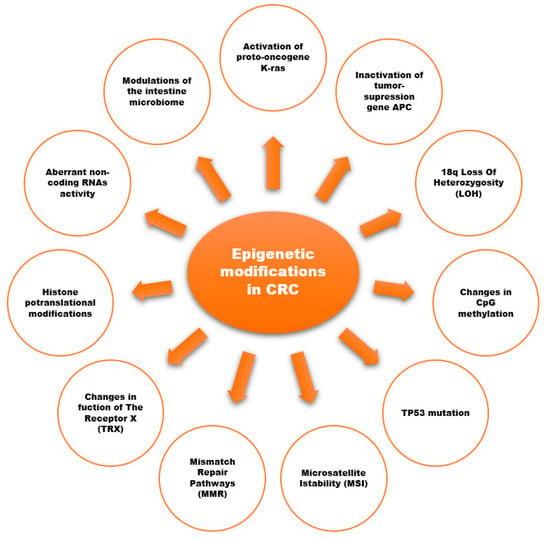
3. Risk Factors for Colorectal Cancer
3.1. Colorectal Cancer Non-Modifiable Risk Factors
3. Risk Factors for Colorectal Cancer
3.1. Colorectal Cancer Non-Modifiable Risk Factors
3.2. Environmental Risk Factors for Colorectal Cancer
3.3. Inherited Genetic Risk for Colorectal Cancer
- (1)
-
Three or more relatives (one of whom is a first-degree relative) with CRC or with HNPCC-s associated cancers (endometrial carcinoma, small bowel adenocarcinoma, ureter or renal pelvis carcinoma);
- Three or more relatives (one of whom is a first-degree relative) with CRC or with HNPCC-s associated cancers (endometrial carcinoma, small bowel adenocarcinoma, ureter or renal pelvis carcinoma);
- (2)
-
Two successive generations affected;
- (3)
-
FAP excluded;
- (4)
-
Tumors confirmed in histology;
- (5)
-
One or more HNPCC-related cancers diagnosed before the age of 50 years.Half of the families that fulfill the original Amsterdam Criteria have a hereditary DNA mismatch repair gene mutation as well as the Lynch Syndrome. The other HNPCC families have no evidence of DNA mismatch repair deficiency, and studies now show that these families are different from Lynch Syndrome families. The name used to refer to the “other half of HNPCC” is Familial Colorectal Cancer Type X (FCCTX), which is undoubtedly a heterogenous grouping [39]. It likely includes some families that have a random aggregation of a common tumor; some families may be attributable to shared lifestyle factors and/or a polygenic predisposition, and some families likely have a yet-to-be-defined syndrome or an undiagnosed single-gene disorder [40].Half of the families that fulfill the original Amsterdam Criteria have a hereditary DNA mismatch repair gene mutation as well as the Lynch Syndrome. The other HNPCC families have no evidence of DNA mismatch repair deficiency, and studies now show that these families are different from Lynch Syndrome families. The name used to refer to the “other half of HNPCC” is Familial Colorectal Cancer Type X (FCCTX), which is undoubtedly a heterogenous grouping [45]. It likely includes some families that have a random aggregation of a common tumor; some families may be attributable to shared lifestyle factors and/or a polygenic predisposition, and some families likely have a yet-to-be-defined syndrome or an undiagnosed single-gene disorder [53].
3.4. COX Role in Colorectal Cancer Carcinogenesis
3.4. COX Role in Colorectal Cancer Carcinogenesis
In recent decades, the role of cyclooxygenase 2 (COX-2) has been appreciated in cancer development and progression. Cyclooxygenase converts arachidonic acid to prostaglandin HIn recent decades, the role of cyclooxygenase 2 (COX-2) has been appreciated in cancer development and progression. Cyclooxygenase converts arachidonic acid to prostaglandin H2. There are two main COX isoforms that are known: the “constitutive” isoform COX-1 and the “inducible” isoform COX-2. Since the early 1990s, there have been many publications confirming that COX-2 promotes pro-tumorigenic activity through several mechanisms: angiogenesis development and resistance to apoptosis, the modulation of host immune surveillance, increasing DNA mutagenesis, activity peroxidase activity and xenobiotic carcinogens, and promoting invasiveness.In CRC, there is an overexpression of the COX-2 protein or mRNA compared to the surrounding normal mucosa. COX-2 overexpression is observed in up to 90% of CRCs. It is interesting that COX-2 expression is increased in adenoma and carcinoma; the COX-2 expression is higher in larger tumors and deep invasions [56,57].In CRC, there is an overexpression of the COX-2 protein or mRNA compared to the surrounding normal mucosa. COX-2 overexpression is observed in up to 90% of CRCs. It is interesting that COX-2 expression is increased in adenoma and carcinoma; the COX-2 expression is higher in larger tumors and deep invasions [41][42].The human COX-2 gene, mapped to chromosome 1q25.2-q25.3, is 8.3 kb in size, contains 10 exons, and produces an mRNA of 4.6 kb. The COX-2 gene is polymorphic, and contains a large number of single-nucleotide polymorphisms (SNPs), such as −765 G>C (rs20417), −1195 G>A (rs689466), −8473 T>C (rs5275), −1759 G>A (rs3218625), −202 C>T (rs2745557), and −1290 A>G (rs689466). COX-2 expression and COX-2 functional polymorphisms are thought to be an early event involved in colorectal cancer development.The human COX-2 gene, mapped to chromosome 1q25.2-q25.3, is 8.3 kb in size, contains 10 exons, and produces an mRNA of 4.6 kb. The COX-2 gene is polymorphic, and contains a large number of single-nucleotide polymorphisms (SNPs), such as −765 G>C (rs20417), −1195 G>A (rs689466), −8473 T>C (rs5275), −1759 G>A (rs3218625), −202 C>T (rs2745557), and −1290 A>G (rs689466). COX-2 expression and COX-2 functional polymorphisms are thought to be an early event involved in colorectal cancer development.4. Colorectal Cancer Treatment—A Multidisciplinary Approach
4.1. Colorectal Cancer Surgery
The contemporary treatment of CRC is based on the combined usage of different methods of therapy. At certain levels of disease advancement, surgical treatment, radiotherapy, and chemotherapy are administrated according to the TNM classification. There is no doubt that surgery remains the gold standard of treatment that allows the optimal goal to be achieved, which is a complete cure for the disease. The location of the tumor in the large intestine and the severity of the neoplastic disease implies the possible method of treatment (surgery, radiotherapy, chemotherapy). The advances in surgical treatment in recent decades allow us to ensure that the most severe surgical complications can be avoided and the continuity of the gastrointestinal tract is maintained. The extent of intestinal resection depends on tumor localization. There are no established standards of management among patients with grade IV, and the method of surgical treatment depends on the goal that is possible to achieve. In small-group patients with the presence of peritoneal metastases as the only cancer dissemination site, hyperthermic intraperitoneal chemotherapy (HIPEC) can also be applied with a chance of success only when it is combined with the maximum cytoreductive surgical procedure (the removal of all tumor sites with a diameter above 0.5–1 cm) and in the absence of distant metastases [43][44].4. Colorectal Cancer Treatment—A Multidisciplinary Approach
4.2. The Length of the Gut Resection Margins
4.1. Colorectal Cancer Surgery
CRC can spread an absorbent through the blood vessels through the continuity and exfoliation of tumor cells. The head principle of oncological surgeons is to not separate/split the macroscopically visible infiltration of the tumor in adjacent organs but to remove it entirely—this strategy is called “en-block resection”. However, it is of huge importance in the case of CRC infiltration that there are no cancer cells in the surgical cut line (R0 resection). It is considered that a proximal and distal intestinal margin of 5 cm in length is enough to ensure the radicality of the procedure [44][45]. This rule, however, does not apply to the distal margin length in the case of rectal cancer’s low-resection: pathomorphological evidence showed that despite the presence of tumor infiltration below the lower tumor border (DIS = distal intramural spread) states in about 2–50% of patients, only in about 5% of cases did the length of infiltration exceed 10 mm. In the case of low resection-located rectal cancer, a distal bowel margin of more than 1 cm was considered to be sufficient (under investigation) [46].The contemporary treatment of CRC is based on the combined usage of different methods of therapy. At certain levels of disease advancement, surgical treatment, radiotherapy, and chemotherapy are administrated according to the TNM classification. There is no doubt that surgery remains the gold standard of treatment that allows the optimal goal to be achieved, which is a complete cure for the disease. The location of the tumor in the large intestine and the severitumber of ly of the neoplastic disease implies the possible method of treatment (surgery, radiotherapy, chemotherapy). The advances in surgical treatment we have seen in recent decades allow us to ensure that the most severe surgical complications can be avoided and the continuity of the gastrointestinal tract is maintained. The extent of intestinal resection depends on tumor localization. There are no established standards of management among patients with grade IV, and the method of surgical treatment depends on the goal that is possible to achieve. In small-group patients with the presence of peritoneal metastases as the only cancer dissemination site, hyperthermic intraperitoneal chemotherapy (HIPEC) can also be applied with a chance of success only when it is combined with the maximum cytoreductive surh nodes found during surgical procedure (the removal of all tumor sites with a diameter above 0.5–1 cm) and in the absence of distant metastases [58,59].4.3. Lymph Node Removal in CRC Surgery
4.2. The Length of the Gut Resection Margins
CRC can sepread an absorbent through the blood vessels through the continuity and exfoliation of tumor cells. The head principle of oncological surgeons is to not separate/split the macroscopically visible infiltration of the tumor in adjacent organs but to remove it entirely—this strategy is called “en-block resection”. However, it is of huge importance in the case of CRC infiltration that there are no cancer cells in the surgical cut line (R0 resection). It is considered that a proximal and distal intestinal margin of 5 cm in length is enough to ensure the radicality of the procedure [59,60]. Thiration is one of the measurable parameters rule, however, does not apply to the distal margin length in the case of rectal cancer’s low-resection: pathomorphological evidence showed that despite the presence of tumor infiltration below the lower tumor border (DIS = distal intramural spread) states in about 2–50% of patients, only in about 5% of cases did the length of infiltration exceed 10 mm. In the case of low resection-located rectal cancer, a distal bowel margin of more than 1 cm was considered to be sufficient (under investigation) [62].4.3. Lymph Node Removal in CRC Surgery
The number of lymph nodes found during surgical preparation is one of the measurable parameters of the f the quality of CRC surgery. There are convincing data that show that, during CRC resection, a minimum of 12 lymph nodes should be removed. However, this recommendation does not apply to rectal cancer previously under radiotherapy or preoperative chemoradiotherapy because lymph nodes may become fibrotic.4.4. Complete Removal of Mesorectum (TME)
4.4. Complete Removal of Mesorectum (TME)
The implementation of the standard of CRC treatment, a new surgical technique involving the complete removal of the mesentery of the rectum (TME, total mesorectal excision), allowed for the reduction in the local recurrence rate after the resection of rectal cancer (from more than 30% to below 10%). Among patients with upper rectum cancer, a full cut-out of the mesorectum at the length of 5 cm below the lower tumor border (subtotal mesorectal excision) is sufficient. A complete mesenteric excision allows us to keep an optimal margin around the tumor. It is known that an excision margin length up to 1 mm is an independent unfavorable prognostic; in such cases, the postoperative pathomorphological report should contain data on both circular margin length and the macroscopic assessment of mesenteric excision according to the so-called Quirck scale [59,60,61][44][45][47].4.5. Colorectal Cancer Systemic Treatment
Therapeutic strategies for rectal cancer have greatly progressed over the last three decades. Preoperative radiotherapy or neoadjuvant chemoradiotherapy (CRT) followed by complete tumor resection (total mesorectal excision—TME) is a standard treatment leading to a reduction in the local recurrence rates in locally advanced rectal cancer. Radiotherapy followed by TME is recommended in intermediate cases (cT2, cT3 without threatened factors, some cT4a) [61,64,65][47][48][49]. In locally advanced cases, and less often in unresectable cases, preoperative chemoradiotherapy followed by radical surgery 6–8 weeks later should be administrated.5. Contemporary Diagnostics and Screening Methods—Guidelines for Colorectal Cancer
5.1. Screening for Colorectal Cancer
5.1.1. Colonoscopy
Colonoscopy is the most well-known and popular screening technique; it has an advantage over other screening methods because of its ability to detect and, at the same time, remove lesions suspected of a neoplastic process [23][50]. It is characterized by very high sensitivity in detecting any visible changes in the large intestine. This applies to both cancer and precancerous lesions. It is worth emphasizing that if no pathological changes are found during the examination, another examination may be performed after 10 years (long intervals). Reports from the USA and Germany highlight the impact of colonoscopy on a reduction in CRC incidence and mortality: 80% in the distal colon and 60% in the proximal colon [67][51].5.1.2. Fecal Immunochemical Tests (FITs)
Fecal immunochemical tests (FITs) for hemoglobin (Hb) are increasingly recommended for colorectal cancer (CRC) screening. An estimated 1–5% of large, tested populations have a positive fecal occult blood test. Of those, about 2–10% have cancer, while 20–30% have adenomas. The advantages of FIT include its non-invasive nature (easy to deliver and affordable), high sensitivity for cancer (79% in 1 meta-analysis), and low costs. The disadvantage of FIT is it needs to be repeated with poor or no sensitivity for serrated class precursor lesions [15,25][11][19].5.1.3. FIT-Fecal DNA Test
The test approved by the FDA (The U.S. Food and Drug Administration) for CRC screening is a combination of FIT and markers for abnormal DNA (aberrantly methylated promoter, regions, mutant K-ras, and β-actin). FIT-DNA has demonstrated a higher sensitivity than FIT for advanced adenomas (42% vs. 23%) and CRC (92% vs. 72%) [67][51].5.1.4. CT Colonography (Virtual Colonoscopy)
CT colonography (CTC) is a tool to evaluate the bowel for CRC for initial bowel screening or after FIT. It requires bowel cleansing preparation. Carbon dioxide is insufflated into the bowel using a small rectal catheter. The advantages of CT colonography include a lower risk of perforation compared with colonoscopy and a sensitivity from 82% to 92% for adenomas >1 cm in size (but for smaller lesions, the sensitivity of CTC drops to 50%). A meta-analysis suggested that symptomatic patients preferred colonoscopy as opposed to screening patients who demonstrated a preference for CTC [25,67][19][51].5.1.5. Flexible Sigmoidoscopy (FS)
FS screens for rectum and sigmoid adenomas use a flexible endoscope inserted into the distal colon. Reductions in the distal colon or rectosigmoid cancer incidence and/or mortality from 29% to 76% with FS have been confirmed through randomized trials. Flexible sigmoidoscopy has several advantages, including lower cost and risk compared with colonoscopy, more limited bowel preparation, and usually no sedation. Flexible sigmoidoscopy is recommended at 5-year intervals [25,67][19][51].5.1.6. Colon Capsule Colonoscopy (CCE)
CCE is approved for average-risk screening and is dedicated to imaging the proximal colon among patients with previous incomplete colonoscopies and for those who need colorectal imaging but have contraindications to sedation. The advantages of capsule colonoscopy are as follows: avoiding invasive procedures and avoiding the risks of colonoscopy. The disadvantage is that bowel preparation is more extensive than that for colonoscopy (European guidelines recommend the use of 4 L of polypethyleneglycol for preparation).5.2. Per Rectum Examination
The oldest and the simplest method for rectum examination is easy to apply in primary care services, with 70% sensitivity for rectal cancer [68][52].5.3. Blood Enzymes Testing
The Septin9 assay is a blood serum assay. SEPT9 is located at chromosome 17q25.3. It is a conservative skeletal protein gene involved in cytokinesis and cytoskeletal organization. SEPT9 is closely related to CRC carcinogenesis when the promoter region is hypermethylated. Once hypermethylated within CRC cells, the septin-9 protein is released into the bloodstream and can be detected via an assay. Sensitivities and specificities for detecting CRC have been reported between 52–73% and 84–91%, respectively. These detection rates were higher for late-stage cancers [25,69][19][53]. The method using the magnetic properties of nanomaterials seems to be promising in this context [70][54]. The CEA tumor marker is an oncofetal antigen generated from endodermal epithelial tumor cells and is primarily used for the tumor detecting and monitoring response to therapy: (1) monitoring patients with CRC, (2) the rapid recognition of the recurrence or spread of CRC, (3) determining the survival time before palliative chemotherapy as an independent prognostic factor, (4) monitoring treatment during palliative chemotherapy, (5) determining the survival time of patients with lymph node metastases as an independent prognostic factor, (6) and the diagnosis of liver metastases (CEA growth > 20 ng/mL is a high probability of metastases in the liver within 3 months) [69,72][53][55].5.4. Recommendations for CRC Screening
The most commonly used methods for CRC screening are a fecal occult blood test, colonoscopy, and sigmoidoscopy, but there is also information on the virtual colonoscopy, magnetic resonance imaging, rectal examination, or rectal enema with double contrast usage in the literature [25,67,68,69][19][51][52][53]. In Europe, the fecal occult blood test (FOBT) performed every year or every 2 years is the most frequently recommended screening test—confirmed as effective and useful in reducing CRC’s mortality rate [74][56]. A positive FOBT result (3–5% of subjects) is obligatorily verified with colonoscopy. To properly perform FOBT, 6 stool samples should be collected (2 specimens from 3 consecutive stools)—a positive result in at least 1/6 of the samples is an indication for a colonoscopy. The disadvantage of this method in comparison to endoscopic methods is undoubtedly its lower sensitivity and specificity in the detection of colorectal cancer, as well as lower sensitivity (11–56%) and zero specificity in the diagnosis of adenomas.6. The Role of General Practitioner and Prophylaxis in Colorectal Cancer Management
The knowledge of CRC risk factors provides opportunities for intervention and early prevention. The leading role in early prevention belongs to primary healthcare [75][57]. It is part of a novel approach to early prevention to start the anticancer battle in the patient’s environment and direct neighborhood. It seems the best place to introduce medicine, a healthy lifestyle approach, information about risk factors, and early symptoms in carcinogenesis is the institution of the General Practitioner (GP). Traditionally, the management of cancer is delivered by in-hospital specialists. In order to provide personalized and integrated care, increase cost-effectiveness, and meet the patient’s needs and expectations, policymakers, patients, and professionals advocate a transfer of cancer care from the hospital environment to the primary care setting. In countries where the GP is the gatekeeper in the care system (e.g., the Netherlands), the GP has a good personal relationship with the patient, with their current state of health and history of previous treatment. The patients often trust their GP in health matters. A good relationship between the GP and the patient has been confirmed in the GRIP study [76][58]. The nature of the GP’s work and working conditions create opportunities to improve the continuous and personalized care of the ever-growing number of cancer patients. All specialists involved in cancer treatment, as well as cancer patients and politicians involved in the healthcare system, point to the extremely significant position of GPs in the period of cancer management and during the follow-up [76][58].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281.
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000.
- Sullivan, B.A.; Noujaim, M.; Roper, J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 177–194.
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254.
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691.
- Tamakoshi, A.; Nakamura, K.; Ukawa, S.; Okada, E.; Hirata, M.; Nagai, A.; Matsuda, K.; Kamatani, Y.; Muto, K.; Kiyohara, Y.; et al. Characteristics and prognosis of Japanese colorectal cancer patients: The BioBank Japan Project. J. Epidemiol. 2017, 27, S36–S42.
- Mahmoud, N.N. Colorectal Cancer: Preoperative Evaluation and Staging. Surg. Oncol. Clin. N. Am. 2022, 31, 127–141.
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516.
- Böckelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2014, 54, 5–16.
- Lawler, M.; Alsina, D.; Adams, R.A.; Anderson, A.S.; Brown, G.; Fearnhead, N.S.; Fenwick, S.W.; Halloran, S.P.; Hochhauser, D.; Hull, M.A.; et al. Critical research gaps and recommendations to inform research prioritisation for more effective prevention and improved outcomes in colorectal cancer. Gut 2017, 67, 179–193.
- Holtedahl, K.; Borgquist, L.; Donker, G.A.; Buntinx, F.; Weller, D.; Campbell, C.; Månsson, J.; Hammersley, V.; Braaten, T.; Parajuli, R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021, 22, 148.
- Vega, P.; Valentín, F.; Cubiella, J. Colorectal cancer diagnosis: Pitfalls and opportunities. World J. Gastrointest. Oncol. 2015, 7, 422–433.
- Hamilton, W.; Sharp, D. Diagnosis of colorectal cancer in primary care: The evidence base for guidelines. Fam. Pract. 2004, 21, 99–106.
- Dunne, J.R.; Gannon, C.J.; Osborn, T.M.; Taylor, M.D.; Malone, D.L.; Napolitano, L.M. Preoperative anemia in colon cancer: Assessment of risk factors. Am. Surg. 2002, 68, 582–587.
- Cai, J.; Yuan, Z.; Zhang, S. Abdominal pain, diarrhea, constipation--which symptom is more indispensable to have a colonoscopy? Int. J. Clin. Exp. Pathol. 2015, 8, 938–942.
- Chardalias, L.; Papaconstantinou, I.; Gklavas, A.; Politou, M.; Theodosopoulos, T. Iron Deficiency Anemia in Colorectal Cancer Patients: Is Preoperative Intravenous Iron Infusion Indicated? A Narrative Review of the Literature. Cancer Diagn. Progn. 2023, 3, 163–168.
- Li, C.; Zheng, H.; Jia, H.; Huang, D.; Gu, W.; Cai, S.; Zhu, J. Prognosis of three histological subtypes of colorectal adenocarcinoma: A retrospective analysis of 8005 Chinese patients. Cancer Med. 2019, 8, 3411–3419.
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 153, 307–323.
- Lieberman, D.A.; Rex, D.K.; Winawer, S.J.; Giardiello, F.M.; Johnson, D.A.; Levin, T.R. Guidelines for Colonoscopy Surveillance after Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012, 143, 844–857.
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic Alterations in Colorectal Cancer. Gastrointest. Cancer Res. 2012, 5, 19–27.
- Chang, S.-C.; Lin, J.-K.; Lin, T.-C.; Liang, W.-Y. Loss of heterozygosity: An independent prognostic factor of colorectal cancer. World J. Gastroenterol. 2005, 11, 778–784.
- Lugli, A. Towards a Molecular Classification of Colorectal Cancer. Front. Oncol. 2015, 5, 46.
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Mediterranean Diet: Prevention of Colorectal Cancer. Front. Nutr. 2017, 4, 59.
- Jia, Y.; Guo, M. Epigenetic changes in colorectal cancer. Chin. J. Cancer 2013, 32, 21–30.
- Harrison, L.E.; Bleiler, M.; Giardina, C. A look into centrosome abnormalities in colon cancer cells, how they arise and how they might be targeted therapeutically. Biochem. Pharmacol. 2017, 147, 1–8.
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213.
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222.
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826.
- Sievers, C.K.; Grady, W.M.; Halberg, R.B.; Pickhardt, P.J. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 723–729.
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 1–15.
- Hughes, L.A.E.; Simons, C.C.J.M.; van Den Brandt, P.A.; van Engeland, M.; Weijenberg, M.P. Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr. Color. Cancer Rep. 2017, 13, 455–469.
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197.
- Bouras, E.; Kim, A.E.; Lin, Y.; Morrison, J.; Du, M.; Albanes, D.; Barry, E.L.; Baurley, J.W.; Berndt, S.I.; Bien, S.A.; et al. Genome-wide interaction analysis of folate for colorectal cancer risk. Am. J. Clin. Nutr. 2023, 118, 881–891.
- Haas, C.B.; Su, Y.-R.; Petersen, P.; Wang, X.; Bien, S.A.; Lin, Y.; Albanes, D.; Weinstein, S.J.; Jenkins, M.A.; Figueiredo, J.C.; et al. Interactions between folate intake and genetic predictors of gene expression levels associated with colorectal cancer risk. Sci. Rep. 2022, 12, 18852.
- DeDecker, L.; Coppedge, B.; Avelar-Barragan, J.; Karnes, W.; Whiteson, K. Microbiome distinctions between the CRC carcinogenic pathways. Gut Microbes 2021, 13, 1–12.
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134.
- Ma, H.; Brosens, L.A.A.; Offerhaus, G.J.A.; Giardiello, F.M.; De Leng, W.W.J.; Montgomery, E.A. Pathology and genetics of hereditary colorectal cancer. Pathology 2018, 50, 49–59.
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, C.R.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014, 147, 502–526.
- Lindor, N.M. Familial Colorectal Cancer Type X: The Other Half of Hereditary Nonpolyposis Colon Cancer Syndrome. Surg. Oncol. Clin. N. Am. 2009, 18, 637–645.
- Poole, E.M.; Bigler, J.; Whitton, J.; Sibert, J.G.; Potter, J.D.; Ulrich, C.M. Prostacyclin Synthase and Arachidonate 5-Lipoxygenase Polymorphisms and Risk of Colorectal Polyps. Cancer Epidemiol. Biomark. Prev. 2006, 15, 502–508.
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101.
- Esquivel, J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: Survival outcomes and patient selection. J. Gastrointest. Oncol. 2016, 7, 72–78.
- Rutkowski, A. Surgical treatment of colorectal cancer in Poland. Clin. Gastroenterol. 2013, 5, 152–161.
- Kkrzakowski, M. Clinical Oncology Volume II. In Gastrointestinal Cancers; Via Medica: Gdańsk, Poland, 2015; pp. 611–642.
- Bujko, K.; Rutkowski, A.; Chang, G.J.; Michalski, W.; Chmielik, E.; Kusnierz, J. Is the 1-cm Rule of Distal Bowel Resection Margin in Rectal Cancer Based on Clinical Evidence? A Systematic Review. Indian J. Surg. Oncol. 2012, 3, 139–146.
- Kozłowska, M.; Głuszek, S. Contemporary methods of treatment of colorectal cancer. Med. Stud. 2015, 4, 307–312.
- Wolpin, B.M.; Mayer, R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology 2008, 134, 1296–1310.e1.
- Van Cutsem, E.; Nordlinger, B.; Cervantes, A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann. Oncol. 2010, 21, v93–v97.
- Hippisley-Cox, J.; Coupland, C. Symptoms and risk factors to identify men with suspected cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2013, 63, e1–e10.
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening—Who, How, and When? Clin. Endosc. 2018, 51, 37–49.
- Świderska, M.; Choromańska, B.; Dąbrowska, E.; Konarzewska-Duchnowska, E.; Choromańska, K.; Szczurko, G.; Myśliwiec, P.; Dadan, J.; Ładny, J.R.; Zwierz, K. Review The diagnostics of colorectal cancer. Contemp. Oncol. 2014, 18, 1–6.
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096.
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585.
- Ma, Z.Y.; Law, W.L.; Ng, E.K.O.; Chan, C.S.Y.; Lau, K.S.; Cheng, Y.Y.; Shin, V.Y.; Kwong, A.; Leung, W.K. Methylated Septin 9 and Carcinoembryonic Antigen for Serological Diagnosis and Monitoring of Patients with Colorectal Cancer after Surgery. Sci. Rep. 2019, 9, 10326.
- Navarro, M.; Nicolas, A.; Ferrandez, A.; Lanas, A. Colorectal cancer population screening programs worldwide in 2016: An update. World J. Gastroenterol. 2017, 23, 3632–3642.
- Cheung, W.Y.; Neville, B.A.; Cameron, D.B.; Cook, E.F.; Earle, C.C. Comparisons of Patient and Physician Expectations for Cancer Survivorship Care. J. Clin. Oncol. 2009, 27, 2489–2495.
- Perfors, I.A.A.; Helsper, C.W.; Noteboom, E.A.; van der Wall, E.; de Wit, N.J.; May, A.M. Randomised controlled trial protocol (GRIP study): Examining the effect of involvement of a general practitioner and home care oncology nurse after a cancer diagnosis on patient reported outcomes and healthcare utilization. BMC Cancer 2018, 18, 132.
