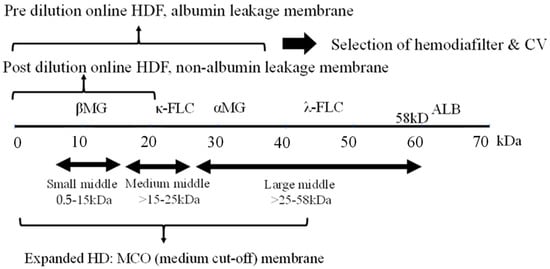
2. Middle Molecules and the Blood Purification Setting (Figure 1)
The removal efficiency of HDF is primarily determined by the volume of replacement fluid, although removal characteristics vary depending on the dilution method employed. Post-dilution HDF, when combined with a protein-non-leakage hemodiafilter, demonstrates efficacy in the removal of small-middle molecules such as βMG. In contrast, for the targeting of medium to larger-middle molecules (e.g., κ-free light chain (FLC) 22 kDa, λ-FLCα1 44 kDa, α1-microglobulin (αMG), 33 kDa), a protein-leakage type hemodiafilter is preferred. However, the drawback of excessive albumin leakage in post-dilution HDF arises due to the challenges in effectively separating albumin from larger-middle substances. In such instances, pre-diluted HDF, capable of discriminating between albumin and larger-middle molecules, is the preferred choice.
Figure 1. Selection of blood purification modality on middle molecules. Definition of middle molecules (MM)s: MWs 0.5 to <58 KD. CV: convection volume, βMG: β2 microglobulin, FLC: free light chain, ALB: albumin.
As a new classification of MM, large pore membrane dialyzers have been used for the removal by HD in the medium to large-middle region
[10][16]. Initially, a high cutoff (HCO) membrane (average pore size 10 nm) was used to remove FLC (κ- 22 kDa, λ- 44 kDa), the causative agent of renal damage due to multiple myeloma, but albumin loss was excessive and it was difficult to use in common HD.
Subsequently, medium cutoff (MCO) membranes (average pore size around 5 nm) were developed and are widely used. Terminology of expanded HD is also used in some cases to indicate that MM removal is more efficient than conventional HD. These MCO membrane dialyzers have about the same performance as protein-leakage filters (dialyzers) in Japan, which means that the super high-flux-albumin leaking dialyzer therapy in Japan is now accepted in Europe, where protein leakage has tended to be denied. Comparisons between this MCO membrane HD and post-dilution HDF have also been made
[11][17], but at present only the name MCO precedes it and no clear criteria have been established. The new definition of MMs requires standards based on a set range of substances to be removed.
3. Impact of Highly Efficient Blood Purification on Patient Survival
3.1. Impact on HDF for Survival
The patient survival outcomes associated with HDF have been subject to extensive review
[5]. The most recent investigations have focused on the survival effects of high CV achieved through post-dilution online HDF in European settings. Randomized controlled trials (RCTs), including the Dutch CONTRAST trial
[12][18], comparing post-dilution HDF versus low flux HD, and the Turkish study
[13][19], ESHOL study
[14][20], and French study
[15][21], which all compare post-dilution HDF versus high-flux HD, have collectively reported that augmenting CV with HDF (with CV values of 22 L
[12][18], 17.2 L
[13][19], 20.7 L
[14][20], and 22.9–23.9 L
[15][21] in each respective study) demonstrates a proportionate enhancement in patient survival.
However, it is essential to note that these RCTs did not explicitly establish CV as a designated treatment objective and did not definitively account for the potential presence of confounding factors. In cases where a higher CV might have been attained, such as in individuals characterized as “healthy” with fewer comorbidities, the presence of native vascular access, and higher blood flow, the risk of mortality was observed to be lower. It is worth acknowledging that these factors were not systematically controlled for in the study design.
Furthermore, the relatively low mean age of the study participants (53 years) and the absence of comprehensive information regarding the selection process and participating centers raised questions about the generalizability of the findings to the broader dialysis population. Consequently, it remained uncertain whether the procedure was universally applicable to all dialysis patients
[16][22]. The theoretical significance of increased solute removal as a determinant of improved life expectancy also warranted further clarification, as no specific upper limit for CV had been proposed.
Conversely, an observational study conducted using Euro-DOPPS 4–5 failed to demonstrate a discernible advantage of HDF
[17][23]. Likewise, an RCT in Australia, comparing post-dilution HDF with a CV of 24.7 L to high-flux HD, did not reveal any substantial survival benefit or improvements in neurological symptoms
[18][24]. It is plausible that the prolonged dialysis duration (5 h) and the favorable survival rates (1-year survival exceeding 90%) in this particular study may have contributed to the absence of differential outcomes.
To address the limitations observed in prior HDF studies, two extensive RCTs were conducted in Europe
[16][22]: the CONVINCE study (Comparison of high-dose HDF with high-flux HD) and the H4RT (High-volume HDF versus High-flux HD Registry Trial). These trials encompassed not only assessments of life expectancy but also explored outcomes related to hospitalization, quality of life, and cost-effectiveness.
In the primary analysis of CONVINCE conducted in 2023, a notable 33% reduction in all-cause mortality over a span of 3 years was reported in the high-dose HDF group (CV > 23 L) comprising 683 patients in comparison to the high-flux HD group with 677 patients. Subsequent sub-analyses highlighted specific benefits, particularly among patients without cardiovascular disease (CVD) or diabetes
[19][25].
Nevertheless, it is essential to acknowledge that this RCT coincided with the COVID-19 pandemic, which could potentially have impacted hospitalization rates and other variables. Ongoing investigations and further analyses are slated for the future to gain a more comprehensive understanding of these findings
[20][26].
3.2. Efficacy of Expanded HD
A meta-analysis examining the efficacy of expanded HD revealed superior clearance of medium to large-middle molecules, such as κ/λ FLC, in comparison to high-flux HD and post-dilution HDF. Additionally, it was associated with reduced albumin loss when contrasted with HDF
[21][31]. However, an equivalent meta-analysis focusing on HDF did not demonstrate a survival advantage for expanded HD
[22][32]. This underscores the continued scarcity of long-term survival evidence for expanded HD, as supported by the limited number of studies or randomized controlled trials available to date. Consequently, additional research is warranted to address this knowledge gap and provide further insights into its potential benefits.
4. The Functional Classification of Dialyzer
The functional classification of dialyzers in Japan was initially established by the JSDT. In 1996, it was categorized into Type I and Type II based on in vitro βMG clearance. Subsequently, in 2006, the classification was expanded to encompass Types I-IV. In 2013, further refinement was achieved with the adoption of Type Ia, Ib, IIa, and IIb classifications, which integrated in vitro albumin sieving coefficient (SC) along with βMG clearance.
An analysis of the JRDR utilizing the 2006 classification framework revealed a significant 35% reduction in 3-year mortality among patients using Type V dialyzers when compared to Type IV dialyzers as the reference group. This result emphasizes the effectiveness of employing the so-called super high-flux membranes
[23][33].
As a consequence of the development of MCO membranes and the increasing adoption of Japanese protein-leak dialyzers, predominantly in Europe since approximately 2015, the Storr classification was introduced. This classification incorporated albumin permeability alongside βMG
[24][38]. This framework aligns with the principles outlined in the 2013 Japanese classification.
In line with this evolution, the extracorporeal blood purification meeting in November 2022 Rome introduced a classification system. This classification categorizes patients based on treatment modality (rather than membrane permeability) into low-flux HD, high-flux HD, and expanded HD, with albumin permeability serving as an additional indicator
[25][39].
Japan has taken a leading role in the development of functional classifications for dialyzers, with Japanese dialyzers exhibiting superior performance in comparison to those from other regions
[23][26][33,40]. It is of paramount importance to determine whether this enhanced performance translates into improved clinical outcomes. A prospective observational cohort study titled “Japanese study of the effects of α1-microglobulin reduction rates on survival; JAMREDS”
[27][41] has recently been initiated. This study aims to investigate the prognosis and occurrence of cardiovascular events in dialysis patients, with the goal of elucidating the theory behind the regeneration of α1-microglobulin’s antioxidant function
[28][42].