1. The Hypocretin/Orexin Peptide and Receptor Family
The hypocretin/orexin system represents an extremely complex neuropeptide network in the CNS
[1][2][21,22]. The seminal papers
[3][4][5][23,24,25] that dealt with the discovery of these ligands and their receptors also demonstrated the hyperphagic
[3][23] and neuroexcitatory activity
[4][24] of orexins and the unique distribution pattern of the system. The orexin/hypocretin system, similarly to melanin-concentrating hormone (MCH)-positive neurons
[6][26], has a well-circumscribed expression in the hypothalamus (
Figure 1)
[4][7][24,27]. Its cell bodies are restricted to the lateral, dorsal, dorsomedial, and perifornical areas, and the whole population does not exceed 50,000–80,000 cells in the hypothalamus. However, its axon terminals reach distant regions, and its receptors are scattered throughout the whole CNS
[5][25].
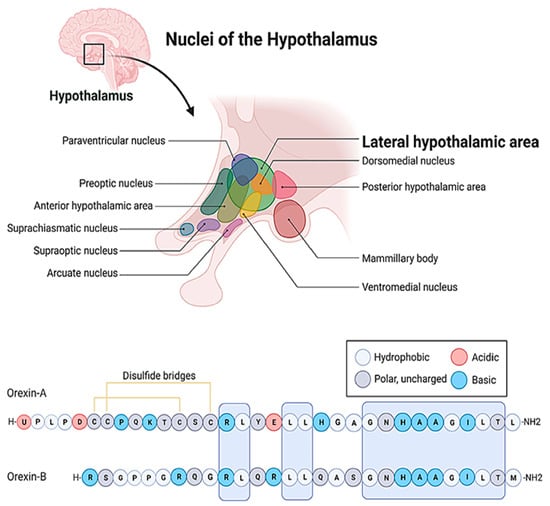
Figure 1. The localization of the lateral hypothalamic area and the amino acid sequences of the orexin/hypocretin family peptides. The letters stand for the one letter code of amino acids. A: Alanine, C: Cysteine, D: Aspartic acid, E: Glutamic acid, G: Glycine, H: Histidine, I: Isoleucine, K: Lysine, L: Leucine, M: Methionine, N: Asparagine, P: Proline, Q: Glutamine, R: Arginine, S: Serine, T: Threonine, U: Pyroglutamic acid, Y: Tyrosine.
At the cellular level, so far, two ligands (orexin-A, orexin-B) and two receptors (OX1R and OX2R) of the system have been described (
Figure 1)
[3][4][23,24]. The peptides biochemically belong to the incretin family, but they bear weak structural resemblance only to a few members of the group
[3][4][23,24]. Even orexin-A and orexin-B differ by 50% of their primary structure. Both peptides are cleft from pre-pro-orexin (PPO) and are amidated C-terminally, but orexin-A is larger, comprising 33 amino acids, while orexin-B consists of only 28 residues
[8][28]. Orexin-A is also less prone to proteolytic degradation because it comprises an N-terminal pyroglutamate residue and two disulfide bonds. Additionally, orexin-A is more hydrophobic, and therefore it can bypass more efficiently the blood–brain barrier (BBB)
[9][29]. These orexins also exhibit significantly different receptor affinities
[3][4][10][23,24,30], which is definitely attributed to the fact that the orexin receptors (OXRs) share only 64% amino acid identity
[8][11][28,31]. The two receptor subtypes create diversity within the cellular signaling pathways
[8][11][12][13][14][28,31,32,33,34]. Both OX1R and OX2R activity is mediated by Gq
11, which, in turn, leads to the activation of phospholipase C (PLC), phospholipase A (PLA), and phospholipase D, ultimately resulting in an increase in cytosolic Ca
2+ and the activation of protein kinase C (PKC). In addition, OX1R can elevate the intracellular Ca
2+ level by activating non-selective cation channels (NSCCs)
[11][31]. On the other hand, OX2R can also inhibit adenyl cyclase (AC) and protein kinase A (PKA) through the G-protein-coupled pathway. The potential dimerization of the OXRs and the structural overlap between OXRs and some other GPCRs lend further diversity to the signal transduction of the system
[11][31]. For example, certain neuropeptide receptors, such as the type-2 neuropeptide-Y (NPY) receptor, the thyrotropin-releasing hormone (TRH) receptor, the cholecystokinin (CCK) type-A receptor, and the NK2 neurokinin receptor, show some similarities (26%, 25%, 23%, and 20% identity, respectively) to the orexin receptors
[3][23]. The highest structural similarity is exhibited by the neuropeptide FF (NPFF) receptor of the RF-amide peptide family, which is 37% identical to OX1R and 35% identical to OX2R, respectively
[15][16][35,36].
Neither the distribution of the immunoreactivity of the two orexins
[17][18][37,38] nor the expression of OX1R
[10][19][20][21][30,39,40,41] and OX2R
[10][20][21][22][30,40,41,42] completely overlaps. This, together with the aforementioned distinct features of the pharmacokinetics of orexin peptides and the differences in the signal transduction of OX1R and OX2R
[12][13][14][32,33,34], must be responsible for some divergence in the physiologic and pathophysiologic actions of orexin-A and orexin-B
[18][38].
2. The Role of Orexins in the Regulation of the Stress Response
The reaction of our neuroendocrine regulation to adverse challenges is provided by the interaction between the sympathoadrenal (SA) system and the HPA axis
[23][124]. Although they represent two distinct pathways, the line between them is frequently blurred, even in scientific literature. Perhaps this is due to their interwoven functions, as they complement each other’s activity while trying to maintain the homeostatic balance of challenged individuals. However, the SA response described by Cannon
[24][125] is carried out according to the cooperation of the autonomic nervous system and the adrenal medulla, while the stress response, discovered by Selye
[25][126], relies on the reaction of the HPA system, one of the central neuroendocrine axes later described by Schally, Guillemin
[26][127], and Vale
[27][128]. Unfortunately, by now, the terminology has been oversimplified, and stress response (though it has several stages) is frequently used as an umbrella term for both responses. Only in meticulous descriptions are these two neuroendocrine reactions clearly separated. To avoid confusion, for the HPA response, the most suitable term is the synonym (general adaptation syndrome: GAS) coined later by Selye
[28][129]. Nonetheless, the distinction between the two pathways is of crucial importance because it helps clarify many contradictions in the literature. Some conflicting responses to certain stress paradigms could be easily resolved by clear discrimination between the two potential targets of adverse stimuli, that is, the SA system and the HPA axis.
It is well known that many neuropeptides modulate the activity of the HPA axis. For instance, NPY, neurotensin (NT), ghrelin, apelin, and endomorphins activate
[29][30][31][32][33][34][35][14,17,20,130,131,132,133] while oxytocin and natriuretic peptides inhibit the system
[36][37][38][39][134,135,136,137]. The output of the HPA axis is quite uniform: it begins with the pituitary translation and cleavage of pro-opiomelanocortin (POMC), yielding adrenocorticotropic hormone (ACTH), which, upon secretion, stimulates glucocorticoid release from the adrenal cortex
[25][28][126,129]. However, in sharp contrast with the output, the input of the HPA axis is extremely diverse and involves a multitude of neuropeptides in signal transduction
[33][131]. Therefore, it is not surprising that the modality (systemic or neurogenic) and schedule (acute, repeated, or chronic) of the stressors strongly influence the extent of the HPA response
[40][138]. Systemic challenges (e.g. osmotic, immune, etc.) perturb the homeostatic balance of the organism, which is directly projected to the brainstem, while neurogenic paradigms (fear, pain) are processed by the cerebral centers
[23][124]. The responses to these two types of challenges are signaled in a dichotomized manner in the brain. The corticotrope-releasing hormone (CRH)-positive neurons of the PVN are responsible for the acute and processed stimuli, while parvocellular arginine vasopressin (AVP) cells in the PVN and the SON maintain responsiveness to chronic, repeated, and homeostatic challenges
[41][139]. It is also worth noting that neuropeptide modulation perfectly complements the built-in brakes of the GAS: the stepwise ultrashort, short, and long loop feedback mechanisms provided by CRH, ACTH, and the glucocorticoids, as well as the potent anti-inflammatory action of the glucocorticoids
[23][124]. These mechanisms are called stress coping or stress resilience, and they harness the severe inflammatory response (SIRS), which otherwise could consume the organism
[23][42][43][124,140,141].
As far as the effect of orexins on the HPA axis and the SA system is concerned, the two responses work hand in hand. Namely, in both responses, orexins play a predominantly stimulatory role
[44][45][80,81]. However, according to the data from the literature, they are stimulated separately. It seems that the SA system is uniformly activated by orexin-A, which stimulates the OX1Rs expressed in the neurons of the nucleus of the solitary tract (NST), the LC, and the sympathetic neurons
[44][46][47][48][49][50][51][53,65,75,80,142,143,144]. Ultimately, it is not a far-fetched idea to state that the perifornical, dorsal, dorsomedial, and lateral hypothalamic foci of orexin-positive neurons can be identified with those in the caudal hypothalamic region, which were demonstrated to be essential for an intact “fight or flight” and “sham rage” response by Philip Bard and Walter Hess
[52][53][8,106].
However, as for the HPA axis, the picture is more complex. Soon after the discovery of the dense orexinergic innervation of the hypothalamic centers (PVN and SON) of the GAS, the scientific rivalry surrounding this highly coveted topic begot several important papers, which established that orexin neurons can activate the HPA axis predominantly at the hypothalamic level
[47][54][55][11,65,119]. The main targets of the orexin neurons are the OX2Rs
[56][145] expressed in the CRH-positive perikarya of the PVN
[5][55][25,119]. Nonetheless, later publications showed that the connection between the orexin- and CRH-positive neuron population is bidirectional since abundant CRH-positive fibers land in the orexinergic perikarya of the hypothalamus
[57][58][59][146,147,148]. Apparently, orexin-evoked HPA activation also involves the release of noradrenaline and NPY
[60][61][62][63][13,149,150,151], which can significantly diversify its processing
[42][43][140,141].
As far as the input of the HPA axis is concerned, the activity of orexins appears to be stressor- and schedule-specific
[44][45][80,81]. In an acute setting, the challenges processed with heightened arousal (aversive odors, novelty, and contextual fear) give rise to more conspicuous activation of the orexin neurons (verified according to
c-fos expression) than systemic challenges (e.g., cold exposure) or long-lasting procedures (e.g., restraint and immobilization)
[44][45][64][65][80,81,108,152]. Nevertheless, while acute stress mostly activates the orexin neurons, experiments with chronic or repeated stressors returned mixed results
[44][45][80,81], the findings of which may reflect an adaptation to unavoidable and permanent challenges. Further studies have revealed that the involvement of the orexins in the GAS depends on not only the modalities and schedule of the applied stressor but also the species and gender of the investigated subjects. Females and strains with better stress resilience phenotypes release less orexin in response to adverse stimuli
[44][45][80,81].
Regarding the output of the HPA axis, orexins have been proven to stimulate the HPA axis not only at the hypothalamic but also at the pituitary and adrenal levels
[44][45][80,81]. This finding is of crucial importance as peripheral activation stabilizes the HPA response to prolonged stimuli. It nurtures sufficient basal activity but also prevents an exaggerated hypothalamic response by maintaining negative feedback through the release of ACTH and glucocorticoids. Apparently, the orexin/hypocretin system also plays a crucial role in the cooperation and seamless integration of the GAS and the “fight or flight” response. Even the earliest publications which dealt with the orexin system demonstrated the dense innervation of the BNST, a limbic center, which harmonizes the activity of the SA system and the GAS
[5][25]. Therefore, it is not unrealistic at all to conceive of orexins as the coordinators of the stress response to challenges with heightened arousal
[44][45][80,81].