Cardiovascular diseases are the leading cause of death worldwide, and arterial hypertension is a recognized cardiovascular risk factor that is responsible for high morbidity and mortality. Arterial hypertension is the result of an inflammatory process that results in the remodeling and thickening of the vascular walls, which is associated with an immunological response. Previous studies have attempted to demonstrate the relationship between oral disease, inflammation, and the development of systemic diseases. The existence of an association between periodontitis and hypertension is a controversial issue because the underlying pathophysiological processes and inflammatory mechanisms common to both diseases are unknown.
- arterial hypertension
- periodontitis
- inflammation
- miRNAs
1. Introduction
2. Hypertension
Hypertension (HT) is a disease that constitutes the main risk factor for cerebrovascular disease and coronary heart disease and accounts for 54% of cerebrovascular diseases and 47% of ischemic heart disease in the world [22]. In Chile, ischemic heart disease is the leading cause of death, followed by cerebrovascular disease (42.8 and 42.7 deaths per 100,000 inhabitants, respectively) [23]. Hypertension is characterized by an increase in pressure inside the blood vessels (arteries) and is defined as systolic blood pressure figures greater than or equal to 140 mmHg and diastolic blood pressure figures less than or equal to 90 mmHg, according to the guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Arterial Hypertension [24]. According to the 2006 Quality of Life and Health Survey, arterial hypertension is the main chronic disease reported in the population [25,26][25][26]. The global prevalence of hypertension was estimated to be 1.13 billion in 2015. This high prevalence of hypertension is consistent throughout the world, regardless of income status; it also becomes progressively more common with advancing age. It is estimated that the number of people with hypertension will increase by 15–20% by 2025, reaching about 1.5 billion [24]. Hypertension can be classified into essential or primary HT, which corresponds to approximately 90% of cases and secondary HT in 10% of patients in whom a correctable cause can be detected. Essential HT is a polygenic disorder influenced by multiple genes or genetic combinations. On this genetic basis, a series of acquired or environmental factors exert a deleterious effect on the development of hypertension. These factors include overweight and obesity, a diet rich in salt and low in potassium, a sedentary lifestyle, alcohol consumption, and stress. The causes of secondary HT are classified as frequent and infrequent. The former includes parenchymal kidney disease, renovascular disease, primary hyperaldosteronism, sleep apnea-hypopnea syndrome, and hypertension induced by drugs, including alcohol. Rare causes include Cushing’s syndrome, hyperparathyroidism, coarctation of the aorta, and several different adrenal dysfunction syndromes [27]. The renin–angiotensin–aldosterone system, in addition to its vascular actions, induces oxidative stress at the tissue level, which specially produces endothelial dysfunction, with a breakdown of the balance between the relaxing factors of the blood vessel (nitric oxide (NO), endothelial hyperpolarizing factor (EDHF)) and vasoconstrictor factors (mainly endothelins), thus configuring the hypertensive pathology. The endothelium exerts a protective function of the arterial wall due to its anti-inflammatory, vasodilation, and antithrombotic properties. When it is dysfunctional, its vasodilator capacity decreases and there is vasoconstriction, arterial stiffness, and vascular inflammation with remodeling, which leads to an increase in total peripheral resistance and, consequently, to an increase in blood pressure [28,29][28][29].3. Hypertension and Inflammation
Currently, the concept of inflammation has evolved in multiple directions to explain the different pathophysiological circumstances in which the histological and functional alteration of an organ occurs. HT is the result of an inflammatory process that includes the remodeling and thickening of the vascular walls and is associated with an immunological response. In this way, the concurrence of inflammatory cells, with forms of innate immunity and adaptive immunity [30], is described along the arterial and venous blood vessels. The immune system, inflammation, and hypertension are interrelated, as the immune system triggers an inflammatory process, in which blood pressure can rise, stimulating organ damage. Cells of the innate immune system produce reactive oxygen species (ROS), such as superoxide and hydrogen peroxide, which are intended to kill pathogens. The long-term process of inflammation increases the production of ROS, causing oxidative stress that leads to endothelial dysfunction. Effector T cells and regulatory lymphocytes, which are part of the adaptive immune system, play an important role in constricting blood vessels in hypertension [3]. It must be borne in mind that inflammation is a tissue process that is made up of a series of molecular, cellular, and vascular phenomena with a defensive purpose against physical, chemical, or biological aggressions [31,32,33][31][32][33]; it currently predominates in the pathophysiology of cardiovascular diseases and, among these, arterial hypertension. The inflammatory process is initiated by a gradient of chemotaxis factors expressed in the forms of intercellular (ICAM-1) and vascular (VCAM-1) adhesion molecules that result in the adhesion of monocytes to the vascular wall and their extravasation into interstitial space and the formation of a cellular assembly of macrophages, neutrophils, basophils, mast cells, and eosinophils. At the same time, the activation of a state of platelet adhesiveness and the formation of fibrin networks that configure a prothrombotic state take place on the intravascular endothelial surface. Innate immunity and adaptive immunity reactions are added to this inflammatory process. In innate immunity, complement activation, acute phase protein expression, and cytokine release take place; while adaptive immunity plays a significant role in the inflammatory response, as it helps to orchestrate the immune response and eliminate pathogens [34,35][34][35]. All of this leads to verifying that inflammation is a complex process, which occurs as a response both to infections and a variety of stimuli that cause tissue damage. If the local acute inflammatory response, which is the immediate reaction to the offending agent, is successful, the offending agent is eliminated, the damage does not spread, there are no systemic manifestations, and the tissue is satisfactorily repaired. On the contrary, if the process did not limit the damage, the initially local acute inflammation is transformed into a systemic inflammatory process that does not induce injury or loss of functionality in the infiltrated tissue, which is a distinctive feature of a low-level chronic inflammation state. In this state, the tissue shows high levels of inflammatory factors and infiltrating immune cells, while not exhibiting structural alterations or loss in its primary functions [30,36,37,38][30][36][37][38]. Recent experimental and clinical information suggests that cardiovascular disease, specifically arterial hypertension, could be the consequence of a low-grade systemic inflammatory process. This picture of systemic inflammation is characterized by an increase in the circulating levels of acute-phase proteins, such as C-reactive protein, cytokines with inflammatory activity, such as tumor necrosis factor alpha (TNF-α), and interleukins such as interleukin (IL)-1 and IL-6, which are considered markers of systemic inflammation in hypertension [34,36,39,40,41][34][36][39][40][41].4. Inflammation and Periodontitis
Another disease that is characterized by low-grade systemic inflammation is periodontitis [42], which is the most common chronic inflammatory disease observed in humans, affecting the supporting tissues of the teeth (periodontal ligament, alveolar bone, and root cementum). In the world, its prevalence averages 33%, being more prevalent in some countries than others and affecting almost half of adults in the United Kingdom and the United States and 60% of those over 65 years of age. Recent estimates from the World Health Organization suggest that periodontitis is found in 5–20% of middle-aged adults (35–44 years) in Europe and up to 40% of older people (65–74 years). It is a major public health problem, causing tooth loss, disability, masticatory dysfunction, and poor nutritional status. Periodontitis compromises speech, reduces quality of life, and is a growing burden on the economy [43,44,45,46,47][43][44][45][46][47]. In Chile, there are few studies with published representative samples that assess the periodontal condition; however, studies show that there is an unfavorable periodontal condition in the population and that signs of periodontal destruction are found in adolescence, which may be one of the causes of the toothless adult population. The prevalence of this periodontal damage increases with age, in the female gender, and in the presence of smoking. In addition, it is distributed in a social gradient, with greater damage in the socially less favored groups, with important influences being family income and parents’ education [48]. Its etiology is multifactorial and it is involved in the etiopathogenesis of alcohol, insufficient diet, lack of exercise, and stress, among others [49,50][49][50]. The first step for the development of periodontal disease is the entry of bacteria, such as Gram-negative anaerobic species or their products to the periodontal tissues, causing damage to them. The bacteria produce virulence factors (e.g., lipopolysaccharide (LPS), lipoteichoic acid) that encounter the epithelial cells of the periodontal sulcus. On the other hand, the cells of the junctional epithelium (EU) produce defensins and proinflammatory cytokines, such as IL-1 and TNF, which increase the caliber of blood vessels and induce the expression of cell adhesion proteins. Subsequently, IL8, another cytokine with an important role, attracts polymorphonuclear cells (PMNs) to the site where the bacteria accumulate. These PMNs in the periodontal sulcus release reactive oxygen species and different enzymes that can cause microscopic tissue damage. Despite this, it is often possible to establish a balance of the immune response that helps resolve the inflammatory process. This immune response controls the microorganisms that accumulate in the periodontal sulcus, silently and without expressing appreciable inflammatory clinical signs. Meanwhile, the inflammatory process progresses, becoming chronic, and the degradation of the supporting tissues begins, causing the formation of the periodontal pocket, a loss of clinical attachment, and bone loss [51,52][51][52]. Among the main inflammatory mediators involved in periodontitis are IL-6 and TNF-α, although other authors also highlight the role of IL-1 and IL-8 [53,54][53][54]. Therefore, in the progression of periodontitis, the local inflammatory response is determined by the concentration of bacteria in the periodontal sulcus [55[55][56],56], the susceptibility of the host [57], unfavorable lifestyles and innate immunity mechanisms, and the release of proinflammatory molecules [58,59][58][59]. These molecules can pass into the systemic circulation and act on distant organs, accentuating an inflammatory state. Higher levels of these molecules are present in patients with periodontitis [60,61][60][61]. Likewise, periodontitis has been proposed as a factor that induces and contributes to low-grade systemic inflammation and vice versa [62,63,64][62][63][64]. Consequently, periodontitis may be an indirect risk factor for cardiovascular disease [65,66][65][66].5. Hypertension, Periodontitis, and Inflammation
Patients with periodontitis have a higher risk of developing cardiovascular disease (CVD), as clinical markers of one pathology are associated with the other. Different studies have demonstrated better endothelial function after periodontal treatment, which is essential for vascular risk in hypertension [67,68][67][68]. Del Pinto et al. mention periodontitis as the main source of low-grade systemic inflammation. The proinflammatory signaling cascade triggered in periodontitis causes hyperpermeability of endothelial cells and the subsequent release of cytokines/interleukins (TNF-α, IL-1, IL-6) into the bloodstream, which affect endothelial function, causing alterations in the vascular structure. In addition, periodontitis has been associated with increased odds of antihypertensive treatment failure, as well as with cerebrovascular disease, coronary heart disease, and chronic kidney disease [69]. Endothelial dysfunction occurs due to the reduction in nitric oxide (NO) production caused by the release into the bloodstream of inflammatory markers such as TNF-α and IL-6. Periodontal treatment reduces systemic inflammation, allowing for the improvement of endothelial function by increasing the bioavailability of NO [16]. A meta-analysis that included 16 studies conducted in the last 15 years shows the possible influence of periodontal diseases on HT. This association can be described by common risk factors or by the diffusion of infectious and inflammatory components of periodontal lesions through the bloodstream, immune response, or glucose and lipid metabolism [70]. Although the most likely explanation for understanding the association between HT and periodontitis could be related to low-grade systemic inflammation (Figure 1), an initial path in the study of the relationship between periodontitis and HT is the possibility of a epigenetic influence, mediated by microRNAs, on the appearance and development of these pathologies; this possibility makes it interesting to study epigenetic regulation, which includes not only DNA methylation and histone modification but also the expression of miRNAs [71], which could play a prominent role in the relationship between the pathologies (Figure 1).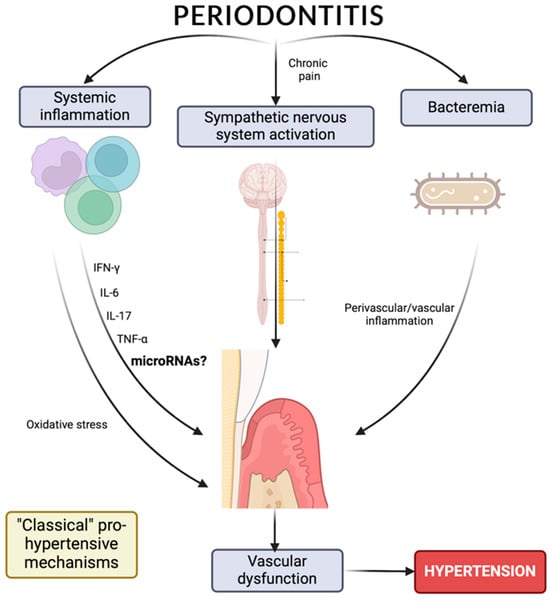
6. MicroRNAs as Risk Biomarkers for Hypertension and/or Periodontitis
miRNAs are small noncoding RNA molecules (19–25 nucleotides) that participate in epigenetic regulation at the posttranscriptional level. Genes that contain miRNAs can be located in intergenic areas, in which the regulation of their expression is produced by their own elements, or in intronic or exonic regions, in which the expression of the miRNA is closely related to the expression of the gene in question. The miRNA can act by degrading the mRNA if there is total complementarity or by repressing translation if the complementarity is partial. Because most of the target sites in the mRNA have only partial base complementarity with each miRNA, the same miRNA can interact with more than 100 different mRNAs. Furthermore, each mRNA can contain multiple binding sites for different miRNAs, giving rise to a complex regulatory network of gene expression. miRNAs have been stably detected in different body fluids, including plasma, and are mainly transported in exosomes or microvesicles, which are associated with proteins (such as Argonaute RISC catalytic component 2 (Ago2) or nucleophosmin 1 (Npm1)), lipoproteins, and even apoptotic bodies. This suggests that circulating miRNAs would be secreted in a regulated manner in response to a stress situation, thus acting as a true intercellular communication system, regulating gene expression and the phenotype of receptor cells. In addition, they can be passively released by damaged or necrotic cells and could not be degraded by RNAses present in plasma [72]. The alteration of the expression values of miRNAs directly affects the expression of their target mRNAs; therefore, miRNAs are considered potentially causative elements of disease. Extracellular miRNAs, like their intracellular forms, are expressed in cardiomyocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells; they control many of the aspects of the biology of the cardiovascular system, such as cardiac remodeling and fibrosis, apoptosis, inflammation, proliferation, angiogenesis, and metabolism. Circulating miRNAs are useful biomarkers in clinical practice due to their biological and physicochemical properties. They are highly stable, can be obtained using minimally invasive techniques, and can originate from necrotic cells or be actively secreted from living cells. The circulating miRNA profile can be highly specific depending on the tissue and the disease; it can also be altered in situations of cellular stress and pathophysiological conditions and have a long half-life within the sample [73]. Alterations in the expression of miRNAs are present in almost all cardiovascular diseases, such as in the development of ventricular hypertrophy, heart failure, and other conditions, including arterial hypertension. An example of this is miR-155, which regulates the expression of the miRNA receptor type 1 angiotensin II, which is positively related to blood pressure [74]; although other miRNAs associated with arterial hypertension have also been reported (Table 1). Similarly, in periodontal disease, miRNAs exert control over aspects of innate and adaptive immunity. As proof of this, miR-15a, miR-29b, miR-125a, miR-146a, miR-148/148a, and miR-223 were found to be upregulated as a result of periodontal disease in both human and mouse studies, while miR -92 was downregulated [75]; this constitutes examples of different miRNAs reported in the literature associated with periodontitis (Table 2). However, to date, there are no miRNA studies that link arterial hypertension with periodontitis.| MicroRNAs | Model | Sample Size | Main Finding | Reference |
|---|
| MicroRNAs | Model | Sample Size | Main Finding | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miR-33a-5p, -144-3p | In vivo | Blood samples from 84 unrelated subjects (42 patients diagnosed with arterial hypertension and 42 normotensive subjects) | Hypertensive patients vs. control group: increased expression of miR-33a (p = 0.001) and miR-144 (p = 0.985), decreased expression of ABCA1 (p = 0.007) and ABCG1 (p = 0.550) transporters. | [76] | ||||||||
| miR-153 | In vivo | Arteries from male normotensive Wistar rats and spontaneously hypertensive rats ranging from 12 to 16 in age | Increased expression of miR 153 in the arteries of spontaneously hypertensive rats (SHR) that exhibited decreased levels of Kv7.4. | [20] | ||||||||
| miR-145 | In vivo and in vitro | Thoracic aortas from 10 26-week-old male spontaneously hypertensive rats and 10 age-matched normotensive male Wistar–Kyoto rats as control group Rat vascular endothelial cells isolated from the thoracic aortas |
miR-145 functions as a key mediator in the pathogenesis of hypertension through SLC7A1 targeting. | [77] | ||||||||
| miR-126 | In vitro | HUVEC cells | Endothelial cells express miR-126, which inhibits VCAM-1 expression. | [78] | ||||||||
| miR-146 | In vitro | THP-1, U937, HL-60, WEHI-3, 293/IL-1R/MD2/TLR4, BJAB, and Mono-Mac-6 cells | The role of miR-146 in the control of toll-like receptor and cytokine signaling through the downregulation of IL-1 kinase 1 and TNF receptor-associated factor 6 protein levels. | [79] | ||||||||
| miR-296-5p, -let-7e, hcmv-miR-UL112. | In vivo | Whole blood from 194 hypertensive patients and 97 healthy volunteers | Twenty-seven differentially expressed miRNAs. Expressions of miR-296-5p, let-7e, and a human cytomegalovirus (HCMV)-encoded miRNA, hcmv-miR-UL112 were validated in plasma samples from 24 hypertensive patients and 22 control subjects. | [80] | ||||||||
| miR-483-3p | ||||||||||||
| In vivo and in vitro | Hearts obtained from age and gender matched transgenic mice over-expressing the human AT1R under the mouse α-MHC promoter and corresponding littermate non-transgenic mouse lines in C3H and C57BL/6 genetic backgrounds (n = 3 for each for group) | Primary human aortic smooth muscle cells |
AT1R-regulated expression levels of angiotensin-1 and angiotensin-1 converting enzyme (ACE-1) proteins in VSMCs are specifically modulated by miR-483-3p. | [83] | ||||||||
| let-7a, -125b, -100, -21 | In vivo | Gingival tissue samples collected from 100 individuals with healthy gingiva and 100 chronic periodontitis patients | Expression analysis revealed that let-7a and miR-21 were upregulated, whereas miR-100, miR-125b, and LIN-28 were downregulated in chronic periodontitis patients relative to healthy individuals. They found that NF-κB was a common target among all four miRNAs. | [19] | ||||||||
| miR-221, -222, -155 | ||||||||||||
| In vitro | ||||||||||||
| let-7a, let-7c, -130a, miR301a, miR-520d and miR-548a, miR-181b, miR-19b, miR-23a, miR-30a, miR-let7a, miR-301a | In vivo | Normal healthy gingiva and diseased gingival tissues obtained from patients undergoing periodontal treatment | miR-let-7a, let-7c, miR-130a, miR301a, miR-520d, and miR-548a were more than 8-fold up-regulated compared to healthy gingiva. MiR-181b, miR-19b, miR-23a, miR-30a, miR-let7a, and miR-301a were successfully amplified and increased significantly more in periodontitis cases than in healthy subjects. | |||||||||
| HUVEC cells | ||||||||||||
| Increased expression of miR-155 and miR-221 in HUVEC and VSMC cells. The angiotensin II type 1 receptor (AT1R) is a target of miR-155 in HUVEC. Ets-1 and its downstream genes, including VCAM1, MCP1, and FLT1, were upregulated in angiotensin II-stimulated HUVECs, and this effect was partially reversed by overexpression of miR-155 and miR-221/2 | [ | 84 | ] | |||||||||
| [ | 103 | ] | ||||||||||
| miR-1274b, -let-7b-5p, -24-3p, -19b-3p, -720, -126-3p, -17-3p, -21-3p. | In vivo | Gingival tissue samples from 9 nonsmoker individuals with chronic periodontitis and 9 nonsmoker individuals with aggressive periodontitis | No differences were observed in the expression profiles of miRNAs between aggressive periodontitis and chronic periodontitis (p > 0.05). The most expressed miRNAs in both groups were hsa-miR-1274b, hsa-let-7b-5p, hsa-miR-24-3p, hsa-miR-19b-3p, hsa-miR-720, hsa-miR-126-3p, hsa-miR-17-3p, and hsa-miR-21-3p. | miR-637 | In vitro | PC12 rat pheochromocytoma cells | ATP6V0A1 expression was affected by differential effects of miR-637, altering vacuolar pH and consequently CHGA processing and exocytotic secretion. | [81] | ||||
| miR-208, -155 | In vivo | Aortas from 8-, 16-, and 24-week-old spontaneously hypertensive rats and 8-, 16-, and 24-week-old normotensive male Wistar–Kyoto rats as control group | The miR-155 level was negatively correlated with blood pressure (r = −0.525, p < 0.05). The expression of miR-208 in the aorta of hypertensive rats was negatively correlated with blood pressure (r = −0.400, p < 0.05) and age (r = −0.684, p < 0.0001). | [85] | ||||||||
| MiR-21, -122, -637, -let-7e | In vivo | Plasma samples from 30 hypertension patients, 30 white coat hypertension patients, and 30 normotensive subjects | The expression levels of MiR-21, miR-122, miR-637, and let-7e were significantly increased in the group of hypertensive subjects compared to the group of normotensive subjects (p = 0.017, p = 0.022, p = 0.048 and p = 0.013, respectively). | [86] | ||||||||
| miR-34a, -21, -126, -146a | In vivo | Plasma samples from 15 normotensive and 15 hypertensive subjects | Circulating expression of miR-34a was higher (~170%; p < 0.01) whereas expression of miR-21, miR-126, and miR-146a were markedly lower (~50%, ~55%, and ~55% respectively; p < 0.05) in the hypertensive versus normotensive. | [87] | ||||||||
| [ | 104 | ] | miR-212, -132 | In vivo | Internal mammary artery with AngII receptor blockers (n = 16) and β-blockers (n = 9) from patients undergoing coronary artery by-pass graft surgery | miR-132 and miR-212 were upregulated in the heart, aortic wall, and kidney of rats with hypertension (159 ± 12 mm Hg) and cardiac hypertrophy after chronic Ang II infusion. In addition, activation of the endothelin receptor, another Gαq-coupled receptor, also increased miR-132 and miR-212. | [88] | |||||
| miR-208b, -133a | In vivo | Blood and urine samples from 102 subjects with untreated newly diagnosed essential hypertension | miRNA-208b and miRNA-133a showed distinct profiles in peripheral blood cells isolated from untreated patients with newly diagnosed hypertension. Their gene expression levels revealed a strong correlation with urinary albumin excretion levels. | [89] | ||||||||
| miR-146a, -155 | In vivo | miR-25, -29a, -26b | In vivo | Blood samples from 104 acute Stanford type A aortic dissection + patients (of which 74 with hypertension and 30 without hypertension), and 103 age-matched acute Stanford type A aortic dissection individuals (of which 59 with hypertension and 44 without hypertension | 4-miRNA (miR-25, miR-29a, and miR-155) were significantly elevated, while miR-26b was decreased in AAAD+ serum samples compared to AAAD individuals), which may serve as a non-invasive biomarker for the diagnosis of AAAD, especially for subjects with hypertension. | [90] | ||||||
| Gingival crevicular fluid from 24 healthy individuals with chronic | periodontitis, 24 patients with chronic periodontitis in association with DM type 2, 24 healthy individuals with clinically healthy periodontium or 24 patients with clinically healthy periodontium in association with DM type 2 | miR-505 | In vivo and in vitro | Peripheral blood from 101 hypertensive patients and 91 healthy volunteers HUVEC cells |
The plasma level of hsa-miR-505 was significantly elevated in hypertensive patients. | [91] | ||||||
| They revealed that miR-146a and miR-155 levels were significantly associated with periodontitis. | [ | 105 | ] | |||||||||
| miR-17 | In vivo | Healthy human tooth samples collected from 8 individuals and teeth affected by periodontal disease collected from seven periodontics clinic patients diagnosed with chronic periodontitis | They found that inflammation resulted in an inhibition of miR-17 levels, which partly reversed the differentiation potential of mesenchymal stem cells (MSCs) isolated from periodontitis-affected periodontal ligament tissue (PDLSC). They confirmed that Smurf1 is a direct target of miR-17 in PDLSC. | [106] | ||||||||
| miR-200b | In vivo | Gingival excess tissue sample was collected from obese and normal weight subjects | The miRNA profile of gingival tissue from obese patients with periodontitis, compared to normal weight patients, showed 13 upregulated and 22 downregulated miRNAs, among which miR-200b was validated by qRT-PCR for significantly increase in obesity. | [107] | ||||||||
| miR-21-5p, -498, -548a-5p, -495-3p, -539-5p, -34c-3p, -7a-2-3p | In vitro | LPS-treated cells from primary human periodontal ligament isolated from explanted healthy periodontal ligament | It was identified 22 upregulated miRNAs and 28 downregulated miRNAs in the LPS-treated periodontal ligament. Seven upregulated (miR-21-5p, 498, 548a-5p) and downregulated (miR-495-3p, 539-5p, 34c-3p, and 7a-2-3p) miRNAs. | [108] | miR-1 | In vivo | Hearts isolated from Wistar rats divided into six groups: control, isoproterenol, ischaemia, ischaemia–propranolol, ischaemia–propranolol-miR-1, and ischaemia–AMO (n | miR-92a | In vivo | Plasma samples from 60 healthy volunteers with normal carotid intima-media thickness (nCIMT), 60 healthy volunteers with increased CIMT (iCIMT), 60 hypertensive patients with nCIMT and 60 hypertensive patients with iCIMT | miR-92a levels showed a significant positive correlation with mean 24-h systolic blood pressure (r = 0.807, p < 0.001), mean 24-h diastolic blood pressure (r = 0.649, p < 0.001), pulse pressure 24-h mean (PP) (r = 0.697, p < 0.001), 24-h daytime PP (r = 0.654, p < 0.001), 24-h nighttime PP (r = 0.573, p < 0.001), CIMT (r = 0.571, p < 0.001) and cfPWV (r = 0.601, p < 0.001). | [92] |
| = 7 for each group) | The beta-adrenergic pathway can stimulate arrhythmogenic miR-1 expression, contributing to ischemic arrhythmogenesis, and beta-blockers produce their beneficial effects in part by downregulating miR-1. | [ | 82 | ] | ||||||||
| 100 | ||||||||||||
| miR-302a-3p | In vitro | ] | ||||||||||
| LPS-treated cells from human mandibular osteoblast-like cells and LPS-treated cells of immortalized normal oral keratinocyte | miR-302a-3p regulates RANKL expression in HMOB within the PGE2 -IFNγ regulatory network. | [ | 109 | ] | ||||||||
| miR-543 | In vitro | Human periodontal ligament-derived stem cells | miR-543 was upregulated during osteogenic differentiation of human periodontal ligament-derived stem cells. Functional experiments showed that overexpression of miR-543 could enhance osteogenesis, while inhibiting miR-543 resulted in reduced formation of mineralized nodules. The ERBB2 transducer, 2 (TOB2) was identified as a target gene of miR-543. | [110] | miR-29a, -29b, -29c | In vivo | ||||||
| miR-664a-3p, -501-5p, -21-3p | In vivo | Serum samples from 30 healthy patients without periodontitis and 30 patients with chronic periodontitis | The expression of hsa-miR-664a-3p, hsa-miR-501-5p, and has-miR-21-3p was higher in the periodontitis group than in the control group (p < 0.05). | [111] | Whole blood from 54 patients with untreated hypertension and 30 healthy individuals | |||||||
| miR-126*, -20a, -142-3p, -19a, -let-7f, -203, -17, -223, -146b, 146a, -155, -205 | In vivo | Gingival tissues obtained from 10 periodontitis patients and 10 healthy subjectshasHsa-miR-126*, hsa-miR-20a, hsa-miR-142-3p, hsa-miR-19a, hsa-lehasf, hsa-has-203, has-miR-17hassa-miR-223, hsa-miR-14has hsa-miR-146a, hsa-miR-155, and hsa-miR-205 showed differential expression levels in subjects with periodontitis in relation to healthy subjects. | [112] | It was observed higher expression levels of miR-29a (p < 0.001), miR-29b (p < 0.001), and miR-29c (p < 0.001) in hypertensive patients compared to healthy control individuals. | ||||||||
| miR-128, -34a, -381, -15b, -211, -372, -656 | In vivo and in vitro | Gingival tissues from periodontitis patients and healthy subjects THP-1 cells and CA9-22 challenged with Porphyromonas gingivalis |
The gingival tissues of patients with periodontitis showed a higher expression of miRNA-128, miRNA-34a, and miRNA-381 and a decrease in the expression of miRNA-15b, miRNA-211, miRNA-372, and miRNA-656. | [113] | [ | |||||||
| miR-150, -223, -200b, -379, -199a-5p, -214. | In vitro | Primary human gingival fibroblasts obtained from patient gingival connective tissue explants | The most overexpressed miRNAs (by >2.72 times) were hsa-miR-150, hsa-miR-223 and hsa-miR-200b, and the three most underexpressed miRNAs (by <0.39 times) were hsa-miR-379, hsa-miR-199a-5p and hsa-miR-214 in inflamed gums of patients with periodontitis. | [114] | 93] | |||||||
| miR-451, -223, -486-5p, -3917, -1246, -1260, -141, -1260b, -203, -210, -205 | - | Data obtained from Gene Expression Omnibus database | Four miRNAs (hsa-miR-451, hsa-miR-223, hsa-miR-486-5p, hsa-miR-3917) were significantly overexpressed and 7 (hsa-miR-1246, hsa-miR-1260, hsa-miR-141, hsa-miR-1260b, hsa-miR-203, hsa-miR-210, hsa-miR-205 *) were underexpressed by >2 times in diseased gums of patients with periodontitis versus healthy gums. | [115] | miR-30e, -374b, -21 | - | Data obtained from Gene Expression Omnibus databasemiR-34b | In vitro and in vivo | ||||
| miR-200c | In vivo and in vitro | 12-week-old male Sprague Dawley rats microinjected with LPS-PG into the gingival sulcus Primary human gingival fibroblasts |
They confirmed that local treatment with miR-200c effectively protected alveolar bone resorption in a rat model of periodontitis, by reducing the distance between the cementum-enamel junction and the alveolar bone crest and the interroot space in the second upper molar. | [116] | It was identified three crucial genes in the hypertensive kidney, such as COL12A1, ASPN, and SCN2A. ASPN could work in conjunction with COL12A1, and both could be targets for miR-21. SCN2A could be a new target for miR-30e and miR-374b. | [94] | ||||||
| miR-9, -126 | In vivo | Peripheral blood mononuclear cells of patients with essential hypertension (n = 60) and of healthy controls (n = 29) for comparison | Hypertensive patients showed significantly lower miR-9 (p < 0.001) and miR-126 (p < 0.001) expression levels compared to healthy controls. | [95] | ||||||||
| miR-425, -155 | In vitro | Human embryonic stem cell-derived cardiomyocytes | The combination of miR-425 and miR-155 reduced NPPA expression to a greater extent than miR-425 or miR-155 alone, regardless of whether they separately also reduced NPPA expression. | [96] | ||||||||
| miR-223 | In vivo | Samples from lean (n = 19) and obese (n = 19) patients | miR-223 mimics the downregulation of TLR4 expression in primary macrophages while downregulating the expression of FBXW7, a well-described suppressor of Toll-like receptor 4 (TLR4) signaling. | [97] | ||||||||
| miR-1, -133a, -26b, -208b, -499, | In vivo | Peripheral blood mononuclear cells from 152 hypertensive patients and 30 healthy volunteers | Hypertensive patients showed significantly lower miR-133a expression levels (p < 0.001) and higher expression levels of miR-26b (p = 0.037), miR-1 (p = 0.019), miR-208b (p = 0.016), miR-499 (p = 0.033) and miR-21 (p = 0.002) compared to healthy controls. | [98] | ||||||||
| miR-17-5p, -106a-5p, -106b-3p, -15a-5p, -15b-5p, -16-5p | In vivo | Case and control pairs (N = 15 pairs) selected from individuals with hypertension treatment | MiRs from the miR-17 and miR-15 families were downregulated in progressive chronic kidney disease with high blood pressure under hypertension treatment compared with appropriate controls. | [99] | ||||||||
| miR-361-5p | In vivo | Whole blood samples from 50 paired hypertensive patients | Significant differences in hsa-miR-361-5p and hsa-miR-362-5p expression levels between samples from patients with salt-sensitive and salt-resistant hypertension (p = 0.023 and 0.049, respectively) | [ | Vascular smooth muscle cells from the medial layer of the thoracic aorta collected from a total of 36 female spontaneously hypertensive and Wistar-Kyoto rats | The negative regulatory association between miR-34b and its target, CDK6, was confirmed, which has potential as a new therapeutic target in the treatment of hypertension. | [101] | |||||
| miR-181a-5p, -27, -125a-5p, -27a-3p, -21-5p, -30a-5p, -98, -92, -22-3p, -100-5p, -99b-5p | In vitro | Human dermal microvascular endothelial cells | Significant suppression of ADM-encoded mRNA expression by endogenous miR-181a-5p, ATP2B1 by miR-27 family, FURIN by miR-125a-5p, FGF5 by let-7 family, GOSR2 by miR-27a-3p, JAG1 for miR-21-5p, SH2B3 for miR-30a-5p, miR-98, miR-181a-5p, and miR-125 family, TBX3 for miR-92 family, ADRA1B for miR-22-3p, ADRA2A by miR-30a-5p and miR-30e-5p, ADRA2B by miR-30e-5p, ADRB1 by the let-7 and miR-98 family, EDNRB by the miR-92 family, and NOX4 by the miR-92 family, miR -100-5p and miR-99b-5p (n = 3–9; p < 0.05 versus scrambled anti-miR). | [102] | ||||||||
References
- Wermelt, J.A.; Schunkert, H. Management of arterial hypertension. Herz 2017, 42, 515–526.
- Solak, Y.; Afsar, B.; Vaziri, N.D.; Aslan, G.; Yalcin, C.E.; Covic, A.; Kanbay, M. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res. 2016, 39, 567–573.
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165.
- Tsounis, D.; Bouras, G.; Giannopoulos, G.; Papadimitriou, C.; Alexopoulos, D.; Deftereos, S. Inflammation markers in essential hypertension. Med. Chem. 2014, 10, 672–681.
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral. Res. 2020, 34 (Suppl. S1), e026.
- Cardoso, E.M.; Reis, C.; Manzanares-Cespedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104.
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Beck, J.D.; Offenbacher, S.; Williams, R.; Gibbs, P.; Garcia, R. Periodontitis: A risk factor for coronary heart disease? Ann. Periodontol. 1998, 3, 127–141.
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 2000, 321, 199–204.
- Devaux, B.; Scholz, D.; Hirche, A.; Klovekorn, W.P.; Schaper, J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur. Heart J. 1997, 18, 470–479.
- Samuels, M.A. Inflammation and neurological disease. Curr. Opin. Neurol. 2004, 17, 307–309.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Lind, L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 2003, 169, 203–214.
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589.
- Engstrom, G.; Stavenow, L.; Hedblad, B.; Lind, P.; Tyden, P.; Janzon, L.; Lindgarde, F. Inflammation-sensitive plasma proteins and incidence of myocardial infarction in men with low cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2247–2251.
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Invest. 2014, 44, 1000–1009.
- Dinh, K.M.; Kaspersen, K.A.; Mikkelsen, S.; Pedersen, O.B.; Petersen, M.S.; Thorner, L.W.; Hjalgrim, H.; Rostgaard, K.; Ullum, H.; Erikstrup, C. Low-grade inflammation is negatively associated with physical Health-Related Quality of Life in healthy individuals: Results from The Danish Blood Donor Study (DBDS). PLoS ONE 2019, 14, e0214468.
- Munoz Aguilera, E.; Leira, Y.; Miro Catalina, Q.; Orlandi, M.; Czesnikiewicz-Guzik, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J. Intern. Med. 2021, 289, 532–546.
- Venugopal, P.; Koshy, T.; Lavu, V.; Ranga Rao, S.; Ramasamy, S.; Hariharan, S.; Venkatesan, V. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell Physiol. 2018, 233, 5877–5884.
- Carr, G.; Barrese, V.; Stott, J.B.; Povstyan, O.V.; Jepps, T.A.; Figueiredo, H.B.; Zheng, D.; Jamshidi, Y.; Greenwood, I.A. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovasc. Res. 2016, 112, 581–589.
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. 2011, 717, 1–8.
- Lawes, C.M.; Vander Hoorn, S.; Rodgers, A.; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518.
- MINSAL Indicadores Básicos de Salud. Available online: https://repositoriodeis.minsal.cl/Deis/indicadores/IBS%202018.pdf (accessed on 26 January 2024).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104.
- MINSAL Resultados I Encuesta de Salud. Available online: http://epi.minsal.cl/wp-content/uploads/2016/03/resumen-ejecutivo-vent.pdf (accessed on 26 January 2024).
- MINSAL II Encuesta de Calidad de Vida y Salud Chile. Available online: https://www.crececontigo.gob.cl/wp-content/uploads/2015/11/ENCAVI-2006.pdf (accessed on 26 January 2024).
- Gijon-Conde, T.; Gorostidi, M.; Camafort, M.; Abad-Cardiel, M.; Martin-Rioboo, E.; Morales-Olivas, F.; Vinyoles, E.; Armario, P.; Banegas, J.R.; Coca, A.; et al. Spanish Society of Hypertension position statement on the 2017 ACC/AHA hypertension guidelines. Hipertens. Riesgo Vasc. 2018, S1889-1837, 30033-3.
- Manfredi Carabetti, J.A. Endotelio, inflamación e hipertensión arterial. Rev. Urug. Cardiol. 2012, 27, 413–417.
- Wagner-Grau, P. Pathophysiology of arterial hypertension. An. Fac. Med. 2010, 71, 225–229.
- Savoia, C.; Schiffrin, E.L. Inflammation in hypertension. Curr. Opin. Nephrol. Hypertens. 2006, 15, 152–158.
- Roitt, I.M.; Brostoff, J.; Male, D.K. Immunology; Mosby: St. Louis, MI, USA, 1998.
- Male, D.C.B.; Cooke, A.; Owen, M. Cell troffic and inflammation. In Advanced Immunology, 2nd ed.; Lippincott: Philadelphia, CA, USA, 1991.
- Rosenberg, H.F.; Gallin, J.I. Chapter 37 Inflammation. In Fundamental Immunology, 6th ed.; Lippincott: Philadelphia, CA, USA, 2003.
- Oliveira, C.M.B.d.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomão, R. Citocinas e dor. Rev. Bras. Anestesiol. 2011, 61, 260–265.
- Pastelin Hernandez, G.; Rosas Peralta, M. Inflammation in high blood pressure. Arch. Cardiol. Mex. 2007, 77 (Suppl. S4), 172–174.
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; Gonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir. Cir. 2015, 83, 543–551.
- Vega, R.G.B. Inflamación. Rev. Fac. Med. UNAM 2008, 51, 220–222.
- Villalba-Herrera, E. Inflamación, I. Rev. Actual. Clínica 2014, 43, 2261–2265.
- Alonso-Rodriguez, D.; Moreno-Tellez, E.; Alarcon-Martinez, Y.; Pedroso-Filiberto, E. Reactive C protein as a marker of inflammation in patients with arterial hypertension. Rev. Med. Inst. Mex. Seguro Soc. 2011, 49, 345–347.
- Herrera Garza, E.H.; Herrera Garza, J.L.; Rodríguez González, H.; Treviño Treviño, A.; Ibarra Flores, M.; Torre Amione, G. Importance of Tumor Necrosis Factor-Alpha in the Pathogenesis of Heart Failure. Rev. Española Cardiol. 2002, 55, 61–66.
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667.
- Rodriguez Coyago, M.L.; Sanchez Temino, V.E. Periodontitis determining the onset and progression of Huntington’s disease: Review of the literature. Medwave 2015, 15, e6293.
- Carvajal, P. Enfermedades periodontales como un problema de salud pública: El desafío del nivel primario de atención en salud. Rev. Clínica Periodoncia Implantol. Rehabil. Oral. 2016, 9, 177–183.
- Moreno-Correa, S.C.-R.A. Molecular mechanisms involved in bone destruction in periodontitis. Literature review. Rev. Clin. Periodoncia Implantol. Rehabil. Oral. 2013, 6, 142–147.
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80.
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez-Aviles, F. European workshop in periodontal health and cardiovascular disease--scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12 (Suppl. B), B3–B12.
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontol. 2000 2017, 75, 45–51.
- MINSAL Protocolo de Referencia y Contrarreferencia para la Especialidad de Periodoncia. Available online: https://www.ssmn.cl/descargas/protocolos_referencia_contrareferencia/hospital_clinico_san_jose/odontologia/Protocolo_Periodoncia.pdf (accessed on 26 January 2024).
- Matos, M.G.; Israel, A.; Billet, E.; Garrido, M.d.R. Citocinas pro-inflamatorias en la enfermedad periodontal experimental: Efecto del valsartán. Rev. Fac. Farm. 2016, 79, 17–27.
- Monzón, J.; Acuña, M.; Caramello, C.; Sesín, J. Periodontitis como factor de riesgo de enfermedades cardiovasculares. Rev. Fac. Odontol. 2017, 10, 32–37.
- Carrillo de Albornoz Sainz, A.; García Kass, A.; Bascones Martínez, A. Papel de la IL-6 y TNF-a en la enfermedad periodontal. Av. Periodoncia Implantol. Oral. 2006, 18, 83–89.
- Botero, J.; Bedoya, E. Determinantes del diagnóstico periodontal. Rev. Clínica Periodoncia Implantol. Rehabil. Oral 2010, 3, 94–99.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8.
- Castro, C.E.; Koss, M.A.; Lopez, M.E. Biochemical markers of the periodontal ligament. Med. Oral. 2003, 8, 322–328.
- Socransky, S.S.; Haffajee, A.D. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: A critical assessment. J. Periodontal Res. 1991, 26, 195–212.
- Contreras, A.; Ramírez, J. Relación entre Periodontitis y Enfermedad Cardiovascular. Rev. Clínica Periodoncia Implantol. Rehabil. Oral. 2009, 2, 91–97.
- Sanz, M.; Quirynen, M.; European Workshop in Periodontology Group A. Advances in the aetiology of periodontitis. Group A consensus report of the 5th European Workshop in Periodontology. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 54–56.
- Heitz-Mayfield, L.J. Disease progression: Identification of high-risk groups and individuals for periodontitis. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 196–209.
- Loos, B.G.; John, R.P.; Laine, M.L. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 159–179.
- Page, R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodontal Res. 1991, 26, 230–242.
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23.
- Teeuw, W.J.; Laine, M.L.; Bizzarro, S.; Loos, B.G. A Lead ANRIL Polymorphism Is Associated with Elevated CRP Levels in Periodontitis: A Pilot Case-Control Study. PLoS ONE 2015, 10, e0137335.
- Gamonal, J.; Acevedo, A.; Bascones, A.; Jorge, O.; Silva, A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J. Periodontol. 2000, 71, 1535–1545.
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 57–71.
- Sima, C.; Glogauer, M. Diabetes mellitus and periodontal diseases. Curr. Diab. Rep. 2013, 13, 445–452.
- Forner, L.; Nielsen, C.H.; Bendtzen, K.; Larsen, T.; Holmstrup, P. Increased plasma levels of IL-6 in bacteremic periodontis patients after scaling. J. Clin. Periodontol. 2006, 33, 724–729.
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470.
- Shrihari, T.G. Potential correlation between periodontitis and coronary heart disease—An overview. Gen. Dent. 2012, 60, 20–24.
- Del Pinto, R.; Ferri, C. Inflammation-Accelerated Senescence and the Cardiovascular System: Mechanisms and Perspectives. Int. J. Mol. Sci. 2018, 19, 3701.
- Martin-Cabezas, R.; Seelam, N.; Petit, C.; Agossa, K.; Gaertner, S.; Tenenbaum, H.; Davideau, J.L.; Huck, O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am. Heart J. 2016, 180, 98–112.
- Cavagnari, B.M. Regulation of gene expression: How do epigenetic mechanisms work. Arch. Argent. Pediatr. 2012, 110, 132–136.
- Fernández-Sanjurjo, M.; Gonzalo-Calvo, D.d.; Díez-Robles, S.; Dávalos, A.; Iglesias-Gutiérrez, E. Circulating microRNA as regulators of the molecular response in exercise in healthy people. Arch. Med. Deport. 2016, 33, 394–403.
- de Gonzalo-Calvo, D.; Iglesias-Gutierrez, E.; Llorente-Cortes, V. Epigenetic Biomarkers and Cardiovascular Disease: Circulating MicroRNAs. Rev. Esp. Cardiol. 2017, 70, 763–769.
- Kaneto, C.M.; Nascimento, J.S.; Prado, M.; Mendonca, L.S.O. Circulating miRNAs as biomarkers in cardiovascular diseases. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2234–2243.
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and immunity in periodontal health and disease. Int. J. Oral. Sci. 2018, 10, 24.
- Huesca-Gomez, C.; Torres-Paz, Y.E.; Martinez-Alvarado, R.; Fuentevilla-Alvarez, G.; Del Valle-Mondragon, L.; Torres-Tamayo, M.; Soto, M.E.; Gamboa, R. Association between the transporters ABCA1/G1 and the expression of miR-33a/144 and the carotid intima media thickness in patients with arterial hypertension. Mol. Biol. Rep. 2020, 47, 1321–1329.
- Wang, Y.; Jin, L. miRNA-145 is associated with spontaneous hypertension by targeting SLC7A1. Exp. Ther. Med. 2018, 15, 548–552.
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521.
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486.
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Lau, W.B.; Rong, R.; Yu, X.; et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011, 124, 175–184.
- Wei, Z.; Biswas, N.; Wang, L.; Courel, M.; Zhang, K.; Soler-Jover, A.; Taupenot, L.; O’Connor, D.T. A common genetic variant in the 3′-UTR of vacuolar H+-ATPase ATP6V0A1 creates a micro-RNA motif to alter chromogranin A processing and hypertension risk. Circ. Cardiovasc. Genet. 2011, 4, 381–389.
- Lu, Y.; Zhang, Y.; Shan, H.; Pan, Z.; Li, X.; Li, B.; Xu, C.; Zhang, B.; Zhang, F.; Dong, D.; et al. MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: A new mechanism for ischaemic cardioprotection. Cardiovasc. Res. 2009, 84, 434–441.
- Kemp, J.R.; Unal, H.; Desnoyer, R.; Yue, H.; Bhatnagar, A.; Karnik, S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J. Mol. Cell Cardiol. 2014, 75, 25–39.
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W.; et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011, 215, 286–293.
- Xu, C.C.; Han, W.Q.; Xiao, B.; Li, N.N.; Zhu, D.L.; Gao, P.J. Differential expression of microRNAs in the aorta of spontaneously hypertensive rats. Sheng Li Xue Bao 2008, 60, 553–560.
- Cengiz, M.; Karatas, O.F.; Koparir, E.; Yavuzer, S.; Ali, C.; Yavuzer, H.; Kirat, E.; Karter, Y.; Ozen, M. Differential expression of hypertension-associated microRNAs in the plasma of patients with white coat hypertension. Medicine 2015, 94, e693.
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Association between hypertension and circulating vascular-related microRNAs. J. Hum. Hypertens. 2018, 32, 440–447.
- Eskildsen, T.V.; Jeppesen, P.L.; Schneider, M.; Nossent, A.Y.; Sandberg, M.B.; Hansen, P.B.; Jensen, C.H.; Hansen, M.L.; Marcussen, N.; Rasmussen, L.M.; et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int. J. Mol. Sci. 2013, 14, 11190–11207.
- Parthenakis, F.I.; Marketou, M.E.; Kontaraki, J.E.; Maragoudakis, F.; Maragkoudakis, S.; Nakou, H.; Roufas, K.; Patrianakos, A.; Chlouverakis, G.; Malliaraki, N.; et al. Comparative microRNA profiling in relation to urinary albumin excretion in newly diagnosed hypertensive patients. J. Hum. Hypertens. 2016, 30, 685–689.
- Xu, Z.; Wang, Q.; Pan, J.; Sheng, X.; Hou, D.; Chong, H.; Wei, Z.; Zheng, S.; Xue, Y.; Zhou, Q.; et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci. Rep. 2017, 7, 13659.
- Yang, Q.; Jia, C.; Wang, P.; Xiong, M.; Cui, J.; Li, L.; Wang, W.; Wu, Q.; Chen, Y.; Zhang, T. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 2014, 177, 925–934.
- Huang, Y.; Tang, S.; Ji-Yan, C.; Huang, C.; Li, J.; Cai, A.P.; Feng, Y.Q. Circulating miR-92a expression level in patients with essential hypertension: A potential marker of atherosclerosis. J. Hum. Hypertens. 2017, 31, 200–205.
- Huang, Y.; Tang, S.; Huang, C.; Chen, J.; Li, J.; Cai, A.; Feng, Y. Circulating miRNA29 family expression levels in patients with essential hypertension as potential markers for left ventricular hypertrophy. Clin. Exp. Hypertens. 2017, 39, 119–125.
- Wang, G.; Wu, L.; Chen, Z.; Sun, J. Identification of crucial miRNAs and the targets in renal cortex of hypertensive patients by expression profiles. Ren. Fail. 2017, 39, 92–99.
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: Potential markers of target-organ damage. J. Am. Soc. Hypertens. 2014, 8, 368–375.
- Vandenwijngaert, S.; Ledsky, C.D.; Agha, O.; Wu, C.; Hu, D.; Bagchi, A.; Domian, I.J.; Buys, E.S.; Newton-Cheh, C.; Bloch, D.B. MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production. PLoS ONE 2018, 13, e0196697.
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962.
- Kontaraki, J.E.; Marketou, M.E.; Parthenakis, F.I.; Maragkoudakis, S.; Zacharis, E.A.; Petousis, S.; Kochiadakis, G.E.; Vardas, P.E. Hypertrophic and antihypertrophic microRNA levels in peripheral blood mononuclear cells and their relationship to left ventricular hypertrophy in patients with essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 802–810.
- Nandakumar, P.; Tin, A.; Grove, M.L.; Ma, J.; Boerwinkle, E.; Coresh, J.; Chakravarti, A. MicroRNAs in the miR-17 and miR-15 families are downregulated in chronic kidney disease with hypertension. PLoS ONE 2017, 12, e0176734.
- Qi, H.; Liu, Z.; Liu, B.; Cao, H.; Sun, W.; Yan, Y.; Zhang, L. micro-RNA screening and prediction model construction for diagnosis of salt-sensitive essential hypertension. Medicine 2017, 96, e6417.
- Yang, F.; Li, H.; Du, Y.; Shi, Q.; Zhao, L. Downregulation of microRNA-34b is responsible for the elevation of blood pressure in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 15, 1031–1036.
- Kriegel, A.J.; Baker, M.A.; Liu, Y.; Liu, P.; Cowley, A.W., Jr.; Liang, M. Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 2015, 66, 793–799.
- Lee, Y.H.; Na, H.S.; Jeong, S.Y.; Jeong, S.H.; Park, H.R.; Chung, J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell 2011, 35, 43–49.
- Amaral, S.A.; Pereira, T.S.F.; Brito, J.A.R.; Cortelli, S.C.; Cortelli, J.R.; Gomez, R.S.; Costa, F.O.; Miranda Cota, L.O. Comparison of miRNA expression profiles in individuals with chronic or aggressive periodontitis. Oral. Dis. 2019, 25, 561–568.
- Radovic, N.; Nikolic Jakoba, N.; Petrovic, N.; Milosavljevic, A.; Brkovic, B.; Roganovic, J. MicroRNA-146a and microRNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J. Clin. Periodontol. 2018, 45, 663–671.
- Liu, Y.; Liu, W.; Hu, C.; Xue, Z.; Wang, G.; Ding, B.; Luo, H.; Tang, L.; Kong, X.; Chen, X.; et al. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells 2011, 29, 1804–1816.
- Kalea, A.Z.; Hoteit, R.; Suvan, J.; Lovering, R.C.; Palmen, J.; Cooper, J.A.; Khodiyar, V.K.; Harrington, Z.; Humphries, S.E.; D’Aiuto, F. Upregulation of gingival tissue miR-200b in obese periodontitis subjects. J. Dent. Res. 2015, 94 (Suppl. S3), 59S–69S.
- Du, A.; Zhao, S.; Wan, L.; Liu, T.; Peng, Z.; Zhou, Z.; Liao, Z.; Fang, H. MicroRNA expression profile of human periodontal ligament cells under the influence of Porphyromonas gingivalis LPS. J. Cell Mol. Med. 2016, 20, 1329–1338.
- Irwandi, R.A.; Khonsuphap, P.; Limlawan, P.; Vacharaksa, A. miR-302a-3p regulates RANKL expression in human mandibular osteoblast-like cells. J. Cell Biochem. 2018, 119, 4372–4381.
- Ge, Y.; Li, J.; Hao, Y.; Hu, Y.; Chen, D.; Wu, B.; Fang, F. MicroRNA-543 functions as an osteogenesis promoter in human periodontal ligament-derived stem cells by inhibiting transducer of ERBB2, 2. J. Periodontal Res. 2018, 53, 832–841.
- Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Fujimori, K.; Sugiura, Y.; Morita, M. Serum microRNAs and chronic periodontitis: A case-control study. Arch. Oral. Biol. 2019, 101, 57–63.
- Xie, Y.F.; Shu, R.; Jiang, S.Y.; Liu, D.L.; Zhang, X.L. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral. Sci. 2011, 3, 125–134.
- Na, H.S.; Park, M.H.; Song, Y.R.; Kim, S.; Kim, H.J.; Lee, J.Y.; Choi, J.I.; Chung, J. Elevated MicroRNA-128 in Periodontitis Mitigates Tumor Necrosis Factor-alpha Response via p38 Signaling Pathway in Macrophages. J. Periodontol. 2016, 87, e173–e182.
- Ogata, Y.; Matsui, S.; Kato, A.; Zhou, L.; Nakayama, Y.; Takai, H. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J. Oral. Sci. 2014, 56, 253–260.
- Stoecklin-Wasmer, C.; Guarnieri, P.; Celenti, R.; Demmer, R.T.; Kebschull, M.; Papapanou, P.N. MicroRNAs and their target genes in gingival tissues. J. Dent. Res. 2012, 91, 934–940.
- Akkouch, A.; Zhu, M.; Romero-Bustillos, M.; Eliason, S.; Qian, F.; Salem, A.K.; Amendt, B.A.; Hong, L. MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators. Stem Cells Dev. 2019, 28, 1026–1036.
