Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Satoru Matsuda and Version 2 by Rita Xu.
Polycystic kidney disease (PKD) is the most common genetic form of chronic kidney disease (CKD), and it involves the development of multiple kidney cysts.
- polycystic kidney disease
- chronic kidney disease
- autophagy
- mitophagy
- mitochondria
1. Introduction
Polycystic kidney disease (PKD) is the most familiar genetic type of CKD. The condition is characterized by the development of multiple kidney cysts, and it could subsequently instigate kidney damage and/or renal failure [1]. This disease may be triggered by mutations in PKD1 or PKD2 genes, which encode the integral membrane proteins polycystin-1 and polycystin-2, respectively [2]. Interestingly, these PKD proteins are associated with decreased autophagy [2][3][2,3]. Autophagy has been found to be impaired in the epithelial cells of the kidneys in animal models of PKD, as well as in patients with PKD, suggesting that this impairment might contribute to the development and/or progression of PKD [4][5][4,5]. In addition, several agents, such as rapamycin, could protect against PKD, which might restore the autophagy in animal models [6]. Mechanistically, aberrant activation of the mammalian target of rapamycin (mTOR), a target molecule of rapamycin, has been shown to be linked to the impaired autophagy as well as the pathology of PKD [7]. Cyst enlargement in PKD kidneys may result in restricted areas of hypoxia [8]. Hypoxic stimuli may then increase the hypoxia-inducible factor-1α (HIF-1α) protein by preventing its degradation by the proteasome. Under hypoxic conditions, the phosphatidylinositol 3-kinase (PI3K) and the mTOR pathway might activate the expression of HIF-1α [9]. (Figure 1) Hypoxia-related events have been revealed to be linked with cyst formation [10][11][10,11]. HIF-1α has been found to be also highly expressed in dendritic cells, and its expression is relatively higher in radicular cysts than in odontogenic tumors [12]. In PKD, HIF-1α may not disturb initial cyst formation, but it is important for cyst progression and expansion in later stages of the disease [13]. Conversely, it has also been shown that HIF-1α inhibition could reduce cystic growth [14]. Signaling pathways being related to the activation of HIF-1α during hypoxia could be contributing to cyst expansion in PKD [15]. Reactive oxygen species (ROS) have been also shown to stimulate cyst development in PKD. In addition, several tissues of PKD may exhibit elevated ROS levels that are positively interrelated with disease severity [15][16][15,16]. Peroxidation of phospholipids in animal and human kidneys may be caused by high amounts of ROS [17][18][17,18]. Cultured renal cysts and MDCK cell cysts in a three-dimensional setup have confirmed a relationship between lipid peroxidation and increased cyst size [14][19][14,19]. It is well-known that damaged mitochondria could induce the generation of ROS and may bring about an increase in membrane lipid peroxidation. Therefore, autophagy, mitochondria, hypoxia and/or ROS might be important administrators in the progression in PKD. Undoubtedly, these hypotheses need further investigation. In addition, despite remarkable efforts to clarify all features of PKD through wide-ranging translational research, there is still an unmet clinical requirement for biomarkers and/or prognosticators that may possibly predict the speed of disease progression [20][21][22][20,21,22]. A better comprehending of the pathophysiology of cystic expansion may lead to the advancement of potential therapies to slow cyst development and/or expansion. The development of innovative treatments that may act synergistically or have fewer side effects might considerably improve the treatment consequences.
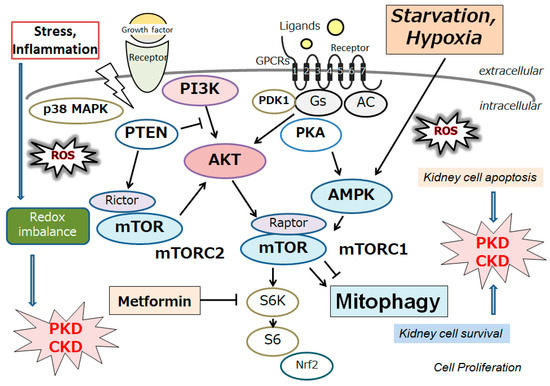
Figure 1. Schematic representation of the relevant signaling pathway potentially being involved in the pathogenesis of polycystic kidney disease (PKD) and/or chronic kidney disease (CKD). Several modulator molecules linked to the PI3K/AKT/mTOR/mTORC1 signaling pathway are demonstrated. Examples of compound metformin, as well as hypoxia and/or starvation, known to act on the AMPK/mTOR and/or mitophagy signaling, are also shown. Arrowhead indicates stimulation, whereas hammerhead shows inhibition. Note that several important activities, such as cytokine-induction and/or inflammatory reactions, have been omitted for clarity. Abbreviation: mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphoinositide-3 kinase; ROS, reactive oxygen species.
2. Autophagy/Mitophagy and Redox Imbalance in the Homeostasis of Kidney Cells
Substantial evidence has supported an imperative role for autophagy in kidney pathophysiology. Autophagy is firmly regulated to support cells to get used to and/or decrease cellular stress. Some studies have emphasized an intricate signaling system that could detect alterations in energy and/or nutrient condition to either activate or prevent autophagy. Intracellular stresses, which can be brought from ROS, hypoxia, stress of endoplasmic reticulum, several DNA damages and/or inflammatory immune signaling have been revealed as potential stimulators of autophagy [23]. Interestingly, autophagy has been defined as a HIF-1α-dependent response [24]. Autophagy describes the process by which cytoplasmic materials, including organelles, access the lysosomes for hydrolytic degeneration [25], which is also a course of cell repair that may frequently convey the apoptosis termed “self-killing” of cells [26]. Dying cells may frequently exhibit an accumulation of autophagosomes and hence adopt a morphology known as autophagic cell death [26]. Consequently, autophagic cell death might cause cell death with autophagy rather than cell death by autophagy. Hypoxia can regulate the mTOR complex 1 (mTORC1) [27]. Therefore, hypoxia and/or mTOR signaling may be modulators of autophagy [27]. (Figure 1) Mitochondria are particularly sensitive to hypoxa, which might result in both functional and morphological impairments. Mitophagy is an arrangement of autophagy that eliminates surplus mitochondria, facilitates reconstruction of mitochondria, and prevents the accumulation of impaired mitochondria [28]. Therefore, mitophagy might be a key mechanism for preserving the quality of mitochondria by eliminating damaged mitochondria. In response to hypoxia, the PTEN-induced putative kinase 1 (PINK1) may be activated as a regulator of mitophagy, confirming the suitable functioning of the total mitochondrial network [29]. Various stressors, such as hypoxia, ischemia, ageing, and oxidative stress, may lead to an increase in ROS and damages to mitochondria, which may trigger the PINK1 mediated mitophagy [30]. (Figure 2) There is evidence for weakened mitophagy in the renal cells of diabetic mice with reduced expressions of mitochondrial PINK1 [31]. A working mitophagy system may act as a scavenger of damaged mitochondria, and thereby maintain a decent mitochondrial homeostasis.
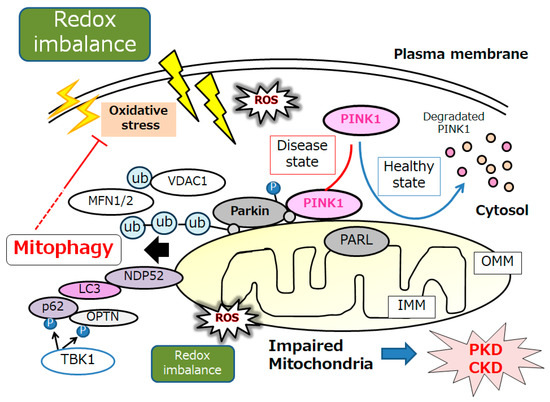
Figure 2. An illustrative representation and overview of PINK1, Parkin, and related molecules in the regulatory pathway for mitophagy. Under the healthy and steady state of cells, PINK1 is despoiled within the surface of mitochondria, which may be reduced by mitochondrial damage due to oxidative stress and/or redox imbalance, resulting in PINK1 and Parkin increases in the outer membrane of mitochondria. Mainly, the PINK1 could phosphorylate ubiquitin to activate the ubiquitin ligase activity for Parkin, where the Parkin is expected to be phosphorylated and ubiquitinated, resulting in the induction of mitophagy. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; MARK2, microtubule affinity regulating kinase 2; MFN1, mitofusin 1; MFN2, mitofusin 2; NDP52, nuclear dot protein 52; PARL, presenilin-associated rhomboid-like; OPTN, optineurin; PINK1, PTEN-induced kinase 1; ROS, reactive oxygen species; VDAC1, voltage-dependent anion channel 1; Ub, ubiquitin.
Mitophagy is principally facilitated by microtubule-associated protein 1 light chain 3 (LC3)-linked receptors. Ubiquitin-dependent mitophagy may include the mitochondrial serine/threonine protein kinase PINK1 and E3 ubiquitin protein ligase Parkin; it also may include the Parkin/PINK1 pathways [32]. Conclusions of the experiment in primary human renal epithelial cells have demonstrated that mitochondrial quality control could be disturbed by mitophagy mediated via PINK/Parkin signaling [33]. PINK1 accumulates on the outer mitochondrial membrane (OMM) after loss of mitochondrial membrane potential, where it recruits and then phosphorylates Parkin to add phosphor-ubiquitin chains on OMM proteins. Interestingly, mitophagy could inhibit oxidative stress via the upregulation of the PINK1-parkin pathway, which could delay kidney senescence in mice [34]. Autophagy receptors, including optineurin, calcium-binding and coiled-coil domain-containing protein 2, also called nuclear dot protein 52 kDa (NDP52), which comprise both ubiquitin binding domains and LC3-interacting regions, could link the ubiquitylated mitochondria to LC3-associated membranes for appropriation [35]. PINK1-mediated phosphorylation of ubiquitin can employ optineurin and/or NDP52 to induce mitophagy without Parkin. By attaching to LC3 at their cytosolic N-terminus, mitophagy receptors could connect impaired mitochondria directly to autophagosomes. (Figure 2) After ubiquitination, impaired mitochondria might be consequently recognized by adapter proteins to be eaten by autophagosomes. Too much mitophagy might result in cellular energy depletion. Therefore, mitophagy may positively or negatively regulate apoptosis, which is a double-edged sword in the pathogenesis of several diseases. For example, a high or low level of mitophagy activity may occasionally induce podocyte apoptosis, which is the collective pathological base for the progression of several kidney diseases [36]. In general, mitophagy may be induced as a protection mechanism for keeping a population of well mitochondria and thus safeguarding cell survival. Although mitophagy may be dispensable for kidney development [37], mitophagy seems to be essential for maintaining kidney integrity and normal physiology in adult kidney cells [38]. Clearance of damaged mitochondria via mitophagy is valuable to the protective effect of impaired kidney cells [39].
3. Autophagy/Mitophagy Involved in the Pathogenesis of Several Kidney Diseases, including Polycystic Kidney
Collecting evidence relates the impaired mitophagy with disease pathogenesis/progression in several pathological situations, including kidney diseases [40]. Acute kidney injury (AKI) may be categorized by a rapid weakening of kidney function, which typically results from renal ischemia, sepsis, and nephrotoxic agents [41]. Mitophagy induction might act as a mutual mechanism to kidney tubular cell protection in many models of AKI [42]. The mitophagy-mediated removal of injured mitochondria might inhibit excessive ROS accumulation, as well as prevent the release of damage-associated molecular arrays which might indorse inflammation during AKI. As renal tissue has massive mitochondrial content, mitophagy and/or mitochondrial biogenesis may be critical to overwhelming stressful illnesses, including AKI [42][43][42,43]. Mitophagy might enable compromised cells to persist during kidney interstitial fibrosis, which is a feature of maladaptive restoration in the transition from AKI to chronic kidney disease (CKD) [44]. Mitophagy being induced in distal tubules and pericytes could protect against renal interstitial fibrosis by suppressing the inflammasome of the tumor growth factor β (TGFβ) and the NLR family pyrin domain containing 3 (NLRP3) signaling [45]. Therefore, mitophagy might be a pharmacological target for the management of interstitial fibrosis in kidneys, particularly in regard to offering new concepts for more efficient anti-fibrosis and delaying the development of CKD [46]. It has been shown that mitophagy activation may protect against renal fibrosis via the downregulation of TGF-β1/Smad signaling, improving mitochondrial fitness and alleviating inflammatory infiltration in kidneys [47]. Focal segmental glomerulosclerosis may be one of the fibrotic diseases in kidneys that is characterized by glomerular lesions with podocytes [48]. It has been revealed that podocyte mitophagy could have an imperative role in the development of the focal segmental glomerulosclerosis [48]. In addition, modifications of the apolipoprotein L1 (APOL1) gene have known links with the focal segmental glomerulosclerosis, which may affect endosomal trafficking and/or block mitophagic flux, eventually leading to podocyte injury [49]. Therefore, podocyte mitophagy could counteract the development of the focal segmental glomerulosclerosis [50]. A decrease in podocyte mitophagy may underlie the conceivable progression of podocytopathies, including the focal segmental glomerulosclerosis [51]. Interestingly, activation of mTORC1 has been detected in glomeruli from patients with the focal segmental glomerulosclerosis [52]. Hyperglycemia may inhibit mitophagy in kidney tubules of diabetic patients with diabetes mellitus [53]. It seems that mitophagy has been impaired in the diabetic kidneys of patients with diabetic kidney disease [53]. Defective mitophagy induced by high glucose levels may accelerate the senescence of tubular cells [54]. Treatment with the mitochondria-targeted antioxidant may ameliorate tubular injury in diabetic mice by restoring mitophagy, which might be mediated by an elevation in PINK1 expression provoked by nuclear factor erythroid 2-related factor 2 [53][54][53,54]. Therefore, mitophagy in kidney tubules might be helpful for diabetic kidney disease.
