Skin diseases represent a global healthcare challenge due to their rising incidence and substantial socio-economic burden. While biological, immunological, and targeted therapies have brought a revolution in improving quality of life and survival rates for certain dermatological conditions, there remains a stringent demand for new remedies. Nature has long served as an inspiration for drug development. Recent studies have identified bitter taste receptors (TAS2Rs) in both skin cell lines and human skin. Additionally, bitter natural compounds have shown promising benefits in addressing skin aging, wound healing, inflammatory skin conditions, and even skin cancer. Thus, TAS2Rs may represent a promising target in all these processes.
- bitter
- TAS2R
- phytochemicals
- skin
- skin inflammation
- skin cancer
1. Introduction
2. Bitter Taste Receptors (TAS2R)-Types and Signaling Pathways
Bitter taste represents one of the five basic tastes (bitter, sweet, sour, salty, umami). From an evolutionary point of view, bitter taste is thought to play an important role in avoiding potentially harmful substances, which most frequently taste bitter [30][31][36,37]. To date, 26 human TAS2Rs are known [32][38]. These receptors belong to the type A G protein-coupled receptor cluster [33][39]. The binding of bitter tastants to TAS2R induces a conformational modification of this receptor, followed by the dissociation of G-protein in Gα-gustducin and Gβγ [34][35][40,41]. Gβγ activates the β2 isoform of phospholipase, capable of converting phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) [34][35][40,41]. Upon binding to its receptor located on the surface of the endoplasmic reticulum (ER), IP3 triggers calcium efflux from the ER, leading to an increase in the cytosolic concentration of Ca2+ [34][35][40,41]. Subsequent molecular events depend on the specific cell type. In the taste buds, a rise in cytosolic Ca2+ concentration determines membrane depolarization through transient receptor potential cation channel subfamily M member 4/5 (TRPM4/5), prompts ATP release from calcium homeostasis modulator 1/3 (CALHM1/3), and initiates the activation of nerve fibers that transmit the gustatory signal to the brain [34][35][40,41]. In the skin, the TAS2R signaling pathway is not yet completely elucidated. Activation of this receptor in skin cells leads to an intracellular increase in Ca2+ concentration [36][42]. Among the 26 known human bitter taste receptors, four are considered orphan receptors (TAS2R42, TAS2R45, TAS2R48, and TAS2R60), indicating that their specific activating compounds or ligands have not yet been identified [37][38][39][40][41][42][43,44,45,46,47,48]. Some of the remaining 22 exhibit broad tuning, displaying varying levels of promiscuity, such as TAS2R10 [37][43], TAS2R14 [37][43][43,49], TAS2R39 [37][43], and TAS2R46 [37][44][43,50]. Others demonstrate narrow modulation, responding to a limited set of agonists; for example, TAS2R3 recognizes chloroquine and theaflavin-3′-O-gallate [37][45][43,51], while TAS2R41 acts as a ‘specialist’ receptor for chloramphenicol [40][46].3. Extraoral Bitter Taste Receptors and Their Biological Roles
TAS2Rs are expressed in virtually all human systems, including cardiovascular [46][47][56,57], digestive [48][49][50][58,59,60], endocrine [51][61], immune [52][53][62,63], integumentary [36][54][55][56][42,64,65,66], muscular [57][67], nervous [58][59][60][61][68,69,70,71], renal [62][63][72,73], reproductive [64][65][66][74,75,76], respiratory [67][68][69][77,78,79], and skeletal systems [70][71][72][73][80,81,82,83]. Evidence shows an increasing number of cells expressing TAS2Rs. Interestingly, recent studies have highlighted differential expression of TAS2Rs in inflammatory versus non-inflammatory states [74][75][84,85], as well as in normal versus cancerous cells [76][77][86,87]. The discovery of extraoral taste receptors has raised questions regarding their biological roles in these cvasi-ectopic locations. Beyond their primordial attribute of detecting bitter taste, TAS2Rs have various non-sensorial functions. They are involved in regulating innate and adaptive immunity [53][78][79][80][81][63,88,89,90,91], inflammation [82][83][31,92], endocrine or exocrine secretion [51][84][85][61,93,94], or the contraction/relaxation process [86][87][95,96]. As well, they are engaged in the pathogenesis of various diseases, such as cancer [66][88][89][90][33,34,76,97], metabolic disorders [91][92][93][98,99,100], and infections [94][95][101,102].4. Differential Expression of Bitter Taste Receptors in Human Skin
The expression of TAS2Rs in human skin varies significantly based on diverse factors such as the degree of sun exposure, physiological characteristics (e.g., sex, age), or the presence of pathological conditions (e.g., inflammation) in the tested specimens. For instance, in sun-exposed skin samples (lower leg) compared to non-exposed areas (suprapubic), TAS2R14, TAS2R30, and TAS2R42 are notably less expressed, while TAS2R60 exhibits a significant increased expression [54][64]. Moreover, a positive correlation occurs in non-exposed skin areas between aging and TAS2R5 expression levels [54][64]. Women display significantly higher expression levels of TAS2R3, TAS2R4, and TAS2R8 in the suprapubic area (considered non-exposed skin), while exhibiting lower levels of TAS2R3, TAS2R9, and TAS2R14 in sun-exposed regions like the lower leg; TAS2R60, however, has a significantly higher expression [54][64]. Scientists also reported high inter-individual variability in TAS2Rs expression, with some having universal expression across individuals, although at varying levels, while others were expressed selectively [54][55][64,65]. In addition, TAS2Rs show differential expression based on the investigated sample types, including whole skin samples, cell lines, or primary cells. For instance, the TAS2R14 transcript had a notably higher expression in human skin samples compared to primary basal keratinocytes and both differentiated and undifferentiated N/TERT-1 keratinocytes [36][42]. Immunofluorescent analysis using anti-TAS2R14 antibodies displayed the strongest signal in the stratum granulosum of skin samples, whereas N/TERT-1 keratinocytes presented a signal dispersed throughout the cytoplasm [36][42].5. Functionality of Bitter Taste Receptors in Skin
There is substantial evidence regarding the functionality of TAS2Rs expressed in the skin. Their activation by corresponding agonists (e.g., (-)-α-thujone for TAS2R14 [36][42], amarogentin for TAS2R1 [56][66]) is associated with a dose-dependent increase in intracellular calcium concentration. Several studies have indicated potential ligands for skin TAS2Rs, suggesting that besides exogenous compounds (both natural and pathogenic) [96][108], endogenous substances could also activate these receptors, including a keratinocyte-derived product not yet identified [97][107] or bitter amino acids derived from natural moisturizing factors [96][108]. Skin TAS2Rs are believed to display diverse biological roles as chemosensory receptors, regulators of keratinocyte differentiation, skin barrier protein expression and lipid synthesis, inhibitors of hair growth, modulators of skin aging, wound healing, adipocyte functions, migration within the skin, and oral microbiota [36][98][99][100][101][42,104,105,109,110].5.1. Bitter Taste Receptors as Chemosensory Receptors
Alongside Merkel cells, keratinocytes have the ability to receive external sensory stimuli and trigger skin sensations, including nociception [102][103][117,118]. There is a suggestion among scientists that TAS2Rs might act as chemosensory receptors in skin cells, allowing them to recognize noxious compounds that may have breached a damaged epidermal barrier.5.2. Bitter Taste Receptors as Regulators of Keratinocyte Differentiation and Skin Barrier Structural and Functional Integrity
Certain bitter compounds, such as amarogentin (a non-selective TAS2R1 agonist) and diphenidol (an agonist for TAS2R1 and TAS2R38), have been found to induce the expression of both early and late differentiation markers in human primary keratinocytes and HaCaT cells (markers including Keratin 10, involucrin, and transglutaminase-1) [56][66]. These compounds not only influence skin barrier proteins but also impact skin lipids.5.3. Influence of Bitter Taste Receptors on Aging and Wound Healing
The aging process in the skin can be induced by D-galactose through various molecular mechanisms involving oxidative stress: downregulation of antioxidant enzymes; formation of advanced glycation end products (AGEs) that target extracellular matrix proteins, such as collagen and elastin, diminish their quality and quantity and subsequently cause reduced skin flexibility; activation of NADPH oxidase; and increase of mitochondrial DNA damage, among others [104][119]. Intriguingly, in D-galactose-induced aged HaCaT keratinocytes, TAS2R10 and TAS2R16, along with downstream proteins (TRPM5 and PLCβ2), had increased expression compared to normal HaCaT cells [99][105].5.4. Bitter Taste Receptors as Regulators of Hair Follicle Growth
The hair follicle growth cycle comprises three phases: 1. anagen, characterized by active cell division and hair growth, lasting 3–10 years; 2. catagen, marked by the cessation of cell division and hair growth, completed in 2–3 weeks; 3. telogen, the resting phase, with a duration of 3–4 months [105][121]. Keratinocytes from the outer root sheath of scalp hair follicles express functional TAS2R4.. Stimulation of TAS2R4 with rebaudioside A has been found to prematurely induce the catagen phase through TGF-β2, thus inhibiting hair growth [98][104].5.5. Bitter Taste Receptors as Modulators of Skin Immunity and Oral Microbiome
Bitter taste receptors play a role in skin immunity. Functional TAS2R38 receptors are expressed by skin-infiltrating lymphocytes [97][107]. Both mRNA and protein expression levels were significantly higher in atopic dermatitis compared to healthy skin [97][107]. This receptor can be considered a marker for the severity of atopic dermatitis based on the positive correlations established between serum thymus and activation-regulated chemokine (TARC), or serum IgE and TAS2R38 mRNA levels [97][107]. Stimulation of TAS2R38 with phenylthiocarbamide (PTC) and 6-n-propylthiouracil (PROP) induces a dose-dependent inhibition of the migration signal in TAS2R38-transduced Jurkat cells in response to CXCL12 [97][107].5.6. Bitter Taste Receptors as Regulators of Adipocyte Functions
Chronic exposure of subcutaneous adipocytes to the bitter compound propylthiouracil increased TAS2R38 expression while concurrently decreasing the expression of three genes involved in adipocyte differentiation (FASN, GLUT4, and PPAR-γ) [106]. In a separate study, two other bitter agonists, denatonium benzoate and quinine, hampered the differentiation of 3T3-F442A pre-adipocytes into mature adipocytes [107][124]. This inhibition resulted in reduced expression levels of several differentiation markers, such as leptin, adiponectin, PPAR-γ, adipocyte protein 2, fatty acid synthase, and uncoupling protein 2 [107][124].5.7. Involvement of Bitter Taste Receptors in Skin and Oral Cancers
5.7.1. Skin Melanoma
In a study conducted by Ryan Carey et al., it was found that among all types of cancer studied, skin melanoma exhibited the highest rate of non-silent mutations for TAS1R and TAS2R [77][87]. Among TAS2R types, the most frequent non-silent mutations involved TAS2R38, TAS2R41, and TAS2R60 [77][87]. This study also investigated TAS1R and TAS2R gene expression and their impact on survival rates [77][87]. Interestingly, increased expression of TAS2R14 was associated with notably longer survival (over 7 years) in distantly metastatic skin melanoma cases [77][87]. Even though melanoma originates from melanocytes [108][125], current genome-wide analysis of tissue-specific RNA and protein expression does not provide evidence of TAS2R expression in melanocytic cells (https://www.proteinatlas.org, accessed on 1, October 2023) [109][126].5.7.2. Oral Squamous Cell Carcinoma
Oral squamous cell carcinoma, much like skin squamous cell carcinoma, originates from keratinocytes but exhibits certain differences in evolution and management [110][111][127,128]. In some samples from oral cavity squamous cell carcinoma and corresponding contralateral normal locations, intraindividual variations in TAS2R expression were observed [112][35]. Differential expressions for TAS2Rs were also observed in oral squamous cell carcinoma cell lines [112][35].5.8. Other Potential Functions of Bitter Taste Receptors
Indirect evidence suggests various potential functions of TAS2Rs. For example, recent research demonstrated the involvement of several TAS2Rs (TAS2R3, TAS2R4, TAS2R14, TAS2R19, and TAS2R43) expressed in follicular granulosa cells in gonadal steroidogenesis [113][129]. Considering the established elevated expression of TAS2R3 and TAS2R4 in the female suprapubic area, an area abundant in sexual hair follicles [54][64], and the recognition of skin and hair follicles as sites for extra-adrenal and extra-gonadal steroidogenesis [114][130], scholars can estimate a potential role for TAS2R3 and TAS2R4 in cutaneous steroidogenesis and hair follicle growth.6. Chemical and Orosensorial Complexity of Bitter Phytochemicals
Bitter phytochemicals are characterized by significant structural heterogeneity, belonging to various chemical classes: alkaloids, aminoacids, carotenoids, coumarins, flavonoids, steroids, terpenoids, etc. [115][53]. The majority of bitter compounds are characterized by their relatively small molecular size and high hydrophobicity, notably distinct from sweet compounds, which are generally larger and more polar [116][54]. A single phytochemical may present a complex orosensory profile, inducing multiple tastes or orosensations. Compounds that display simultaneous bitter taste and astringency are often found in the tannin class (e.g., castalagin activates TAS2R7 [117][131]) or within flavonoids (e.g., myricetin activates TAS2R14 and TAS2R39 [118][132]). Camphor, a well-known pungent monoterpenoid, also activates several bitter taste receptors: TAS2R4, TAS2R10, TAS2R14, and TAS2R47 [37][43]. Although the majority of saccharides are sweet, a few display bitterness (e.g., gentiobiose [119][133], gentianose [120][134], beta-D-mannose) [121][135]). Rebaudiosides, belonging to the class of steviol glycosides, are well known as sweet compounds, but many of them also exhibit slight bitterness, acting as agonists for various bitter taste receptors (e.g., rebaudiosides A, B, and C are agonists of TAS2R4 and TAS2R14 [122][136]).7. Bitter Phytochemicals Active on Skin Inflammation, Skin Carcinogenesis, and Wound Healing
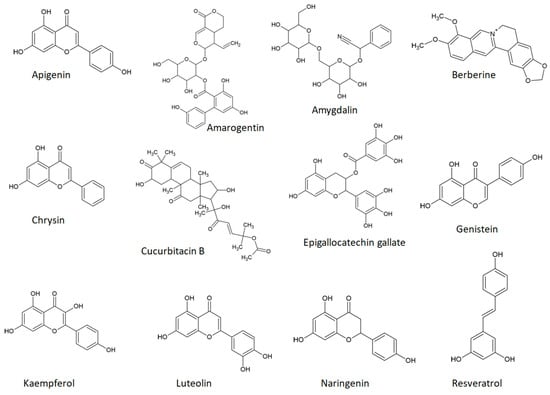
Recently, scholars have demonstrated that taste serves as a more significant predictor of anti-inflammatory and anti-cancer activity than the chemical class itself [28][29][123][28,29,137]. Bitter taste was positively correlated, while sweet taste showed a negative correlation with both activities [29]. As outlined in a recent review, anti-inflammatory activity emerged as the most frequently cited biological property of natural compounds beneficial in wound healing [124][138]. This finding is reasonable considering that inflammation represents the second phase of the wound healing process [124][138]. Therefore, bitter phytochemicals show promising therapeutic potential in treating skin inflammatory diseases, skin cancer, and skin ulcers. Figure 1 illustrates the chemical structures of a select group of bitter phytochemicals, which will be briefly discussed in connection with TAS2Rs.