Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Md Abdus Sattar and Version 2 by Catherine Yang.
Adipose stem cells (ASCs) have multilineage differentiation capacity and hold great potential for regenerative medicine. Compared to bone marrow-derived mesenchymal stem cells (bmMSCs), ASCs are easier to isolate from abundant sources with significantly higher yields. It is generally accepted that bmMSCs show age-related changes in their proliferation and differentiation potentials, whereas this aspect is still controversial in the case of ASCs.
- ASC
- osteogenic potential
- osteoblast
- aging
1. Introduction
Human bone tissue can regenerate spontaneously after fracture or injury through a bone remodeling process consisting of four phases (Figure 1). Within the first five days after injury, the fracture hematoma is formed accompanied by local inflammation, resulting in the activation and migration of immune cells, including macrophages, monocytes, and lymphocytes [1][2][1,2]. Next, the regenerating cells of the connective tissue, including mesenchymal stem cells (MSCs), are recruited to the site of injury, and the fibrocartilaginous fracture callus is formed by the rapid proliferation and differentiation of MSCs into chondroblasts, osteoblasts, and fibroblasts. The differentiation of MSCs is induced by several mediators of cell differentiation such as bone morphogenic proteins (BMPs). The expression and localization of BMPs (e.g., BMP-2 and BMP-4) during early fracture healing have been reported in the literature [3][4][3,4]. The vascular endothelial growth factors (VEGFs) secreted by immune cells promote angiogenesis through the formation of new blood vessels. The third phase involves the endochondral ossification of the cartilage where soft cartilage is replaced by a hard bone callus. VEGF-mediated vascularization continues deeper into the callus and facilitates further migration of MSCs and the supply of oxygen and nutrition. Finally, the newly formed bone callus is continuously remodeled by the resorption of osteoclasts and the formation of osteoblasts [1][5][6][1,5,6].
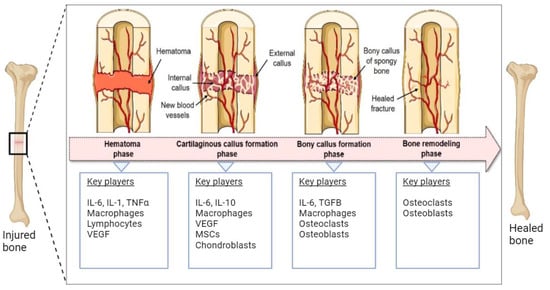
Figure 1. Schematic representation of the natural bone repair process. Immune cells associated with hematoma are formed in the first phase. In the second phase, MSCs are recruited and differentiated into chondroblasts and osteoblasts that help the formation of soft cartilage callus. VEGFs promote vascularization in this phase. In the third phase, soft cartilage is replaced by hard bone facilitated by osteoblasts, osteoclasts, chondroclasts, and chondroblasts. The final step involves the continuous remodeling of newly formed bone. The figure was modified from Pfeiffenberger et al. [1], licensed under a Creative Common Attribution (CC BY) 4.0 Generic License, https://creativecommons.org/licenses/by/4.0/) (accessed on 24 November 2023). Created with BioRender.com (accessed on 24 November 2023).
This spontaneous bone healing is a multilateral process that is regulated by various intrinsic and extrinsic factors and can be disrupted by various causes at various time points, resulting in a failure to heal successfully [7]. Modern surgical techniques augment natural as well as impaired healing by grafting autologous, allogenic, or prosthetic materials to the recipient site [8]. However, the transfer of autologous material is limited by the availability of appropriate tissue, and it holds the risk of donor-site morbidity, unpredictable graft absorption, infections, and structural failure.
To address these issues, bone repair through osteogenic tissue engineering could combine suitable progenitor cells with appropriate scaffolds and growth factors [2]. Autologous stem cell transplantation includes cell isolation and the subsequent in vitro expansion, eventually followed by the transplantation into the defect site. However, this strategy is challenged by the availability of suitable stem cell populations with sufficient intrinsic potential for osteogenic differentiation. Admittedly, age-related changes in stem cell characteristics are an important criterion that should be taken into account when considering autologous stem cell therapy for bone tissue regeneration and/or artificial bone tissue engineering. Although the clinical applicability of MSCs spans all age groups, elderly people are the primary beneficiaries of stem cell-based regenerative medicine because degenerative bone diseases, and delayed or impaired fracture healing are more prevalent in the elderly population. In addition, the risk for bone deformation and fractures per se increases with age, while it is associated with a decreased ability for tissue regeneration and repair. Age-associated microenvironmental changes, such as metabolic alteration, hormonal disturbance, and immunological disorders, might also affect the stem cell niche and, thus, impair the regenerative potential of the MSCs [9]. Moreover, aging induces profound changes in various molecular, genetic, and epigenetic processes, resulting in alterations to the proliferation and differentiation potential of MSCs. Ultimately, this leads to disrupted tissue homeostasis and impaired repair abilities [10]. Therefore, it is not only important to find a suitable autologous cell population with the potential to regenerate bone tissue but also crucial to choose an MSC type that is less affected by age. The most promising candidates are adipose stem cells (ASCs) and MSCs derived from bone marrow (bmMSCs), members of the MSC family, which share unique features for osteogenic differentiation [11][12][11,12].
bmMSCs have multilineage differentiation potential, and their use in regenerative medicine is not restricted by ethical issues. However, as discussed in previous reviews [13][14][15][16][17][13,14,15,16,17], several disadvantages restrict the use of bmMSCs in bone tissue engineering. For example, bmMSC isolation procedures can be associated with donor-site morbidities such as pain, infection, hematomas, seromas, nerve injuries, arterial injuries, and fractures [18]. In addition, aging negatively impacts bmMSC harvests because the cell yield declines with age [19]. Moreover, a negative age effect is observed in both the proliferation as well as osteogenic differentiation potential of bmMSCs [19][20][21][22][19,20,21,22]. Additionally, bmMSCs isolated from elderly donors exhibit increased senescence properties and ROS-induced oxidative damage [20][21][22][20,21,22]. It has been postulated that the bmMSCs from elderly donors favor adipogenic differentiation instead of osteogenic differentiation, a process termed the “adipogenic switch” [13][14][17][13,14,17].
2. Human Adipose Stem Cells in Bone Tissue Engineering
Age-related changes in the osteogenic potential of hASCs might be visible when hASCs from very young and very old donors with distinct age differences are compared. As younger individuals are less likely to undergo surgery, hASCs from young donors are significantly harder to recruit for experimental studies. This might be the reason why previous studies did not investigate hASCs from distinct age groups. For the same reason, hASCs were obtained predominantly from females rather than males because females are more likely to undergo plastic surgery [23][53]. Interestingly, out of the six studies that recruited female donors only, three studies considered age range related to the perimenopause (40–50 years) and revealed that hASCs obtained from female individuals in their early 40s exhibited increased lipid accumulation and decreased potential to differentiate into osteogenic lineage compared to hASCs from younger (<30 years) and older (>55 years) women [24][25][26][27][35,40,47,54]. These authors postulate that menopause-related changes in estrogen levels could explain this transient effect of age on hASC function. It is well known that a declined estrogen level is associated with low-grade inflammation that triggers fat accumulation and activates osteoclasts to degrade bone tissue [28][55]. In conclusion, gender and menopausal status should be considered when grouping donors based on age, and future studies should further explore the effects of hormonal changes and osteoporosis on ASC properties.
Some authors reported high intragroup variability in hASC characterization and differentiation, which could conceal age-dependent effects This apparent high donor-to-donor variability could be attributed to other demographic and lifestyle factors, e.g., general health status, medical and disease history, body mass index, or epigenetic patterns related to the environment; donor habits may also influence experimental outcomes, as reviewed by Prieto González in 2019 [29][56]. These donor characteristics have been disregarded in the literature, and in many cases, BMI was used as the sole parameter to describe the obesity status of individuals [10][24][26][30][31][32][33][34][10,35,36,37,41,43,44,47]. Increased BMI as a marker of obesity is associated with a decreased osteogenic potential of hASCs [35][57]. However, the role of BMI in identifying people with obesity is controversial as it cannot distinguish fat from muscle or bone mass. Therefore, a more useful indicator of obesity should be used when defining non-obese donors of ASCs.
Studies evaluating the effect of age on bone tissue engineering used hASCs from diverse anatomical sites, including the abdomen, the epididymis, and the eyelid. Surgical methods of fat harvesting also varied between the presented studies. Differences in the anatomical origin of adipose tissue and surgical procedures may be the underlying confounder since hASCs from different donor sites and methods of extraction exhibit distinct biological properties [36][58]. For instance, Requicha et al. assessed the expression profile of osteogenic genes (COLIA1, RUNX2, and Osteocalcin) of hASCs from the canine subcutaneous and omental origin by RT-PCR analysis. While RUNX2 expression did not differ between the two fat depots, COLIA1 had significantly higher expression in subcutaneous hASCs, whereas osteocalcin displayed an inverse expression pattern [37][59].
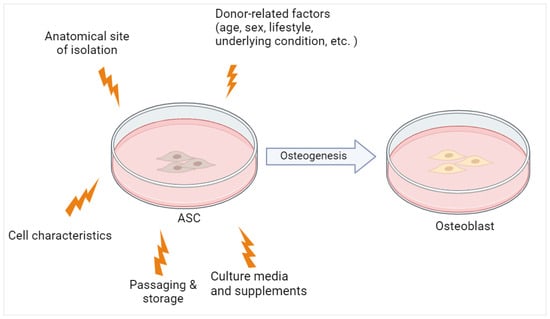
Apart from donor-related factors, the proliferation as well as differentiation potential of hASCs are also influenced by long-term passage, cryopreservation, and culture conditions (Figure 23), as these parameters varied in previous reports [29][38][56,60]. Often, osteogenic induction was performed on cryopreserved cells, after passages 1 to 5 by using osteogenic induction media with different compositions. Furthermore, previous studies selected different endpoints as the marker of osteogenic differentiation with various readout methods. Growth factors and serum supplementation also greatly differ between laboratories. These experimental variations may influence hASC stemness, proliferation, and differentiation [29][56].
 As mentioned before, an accurate definition of ASCs remains an open issue. Among the 15 included studies, 8 studies characterized ASCs based on 2006 ISCT criteria of antigen expression profile for MSCs (Table 12), while 7 studies characterized ASCs by plastic adherence property and tri-lineage differentiation capacity. These characteristics are common for all MSCs and therefore a distinction between ASCs and other MSCs was not apparent. None of the studies included specific markers of ASCs in SVF (CD31−, CD45−, CD235a−, and CD34+) and in culture (CD36+ and CD106−) jointly proposed by the IFATS and ISCT in 2013. Thus, a lack of a uniform method of characterization of ASCs could contribute to conflicting results in previous studies.
As mentioned before, an accurate definition of ASCs remains an open issue. Among the 15 included studies, 8 studies characterized ASCs based on 2006 ISCT criteria of antigen expression profile for MSCs (Table 12), while 7 studies characterized ASCs by plastic adherence property and tri-lineage differentiation capacity. These characteristics are common for all MSCs and therefore a distinction between ASCs and other MSCs was not apparent. None of the studies included specific markers of ASCs in SVF (CD31−, CD45−, CD235a−, and CD34+) and in culture (CD36+ and CD106−) jointly proposed by the IFATS and ISCT in 2013. Thus, a lack of a uniform method of characterization of ASCs could contribute to conflicting results in previous studies.

Figure 23.
The osteogenic potential of ASC is influenced by both donor-related and experimental factors. Created with
(accessed on 24 November 2023).
Table 12.
ASC characterization methods described in the included studies.
| Method | Positive Markers | Negative Markers | References |
|---|---|---|---|
| Flow Cytometry | CD44, CD90, CD105, CD146 | CD3, CD4, CD11b, CD34, CD45 | [10] |
| Flow Cytometry | CD44, CD73, CD90,CD105 | - | [39][38] |
| Flow Cytometry | CD44, CD73, CD90, CD105 | CD3, CD14, CD19, CD34, CD45 | [40][39] |
| Flow Cytometry | CD73, CD90, CD105 | CD14, CD19, CD34, CD45 | [32][41] |
| Flow Cytometry | CD44, CD73, CD90, CD105 | CD34, CD45 | [41][42] |
| Flow Cytometry | CD44, CD73, CD90, CD105 | CD34, CD11b, CD19, CD45, HLA-DR | [33][43] |
| RT-qPCR | CD44, CD73, CD90, CD105, CD271, NANOG | - | [34][44] |
| Flow Cytometry | CD44, CD73, CD90, CD105 | CD31, CD34, CD45 | [42][46] |
