Gastric outlet obstruction (GOO) poses a common and challenging clinical scenario, characterized by mechanical blockage in the pylorus, distal stomach, or duodenum, resulting in symptoms such as nausea, vomiting, abdominal pain, and early satiety. Its diverse etiology encompasses both benign and malignant disorders. The spectrum of treatment modalities extends from conservative approaches to more invasive interventions, incorporating procedures like surgical gastroenterostomy (SGE), self-expandable metallic stents (SEMSs) placement, and the advanced technique of endoscopic ultrasound-guided gastroenterostomy (EUS-GE). While surgery is favored for longer life expectancy, stents are preferred in malignant gastric outlet stenosis. The novel EUS-GE technique, employing a lumen-apposing self-expandable metal stent (LAMS), combines the immediate efficacy of stents with the enduring benefits of gastroenterostomy.
- endoscopic ultrasound-guided gastroenterostomy
- gastric outlet obstruction
- endosonography
- self-expandable metal stent
- gastroenterostomy
1. Introduction
2. Surgical Gastrojejunostomy
This method was considered the traditional bypass method to alleviate obstructive symptoms of GOO and ameliorate patients’ quality of life before the emergence of less invasive endoscopic techniques. It can be performed either as an open or a laparoscopic surgery. It is important that the gastrostomy is formed 3–5 cm proximal to either the obstruction or the pylorus. The anastomosis needs to be placed at the distal stomach on the greater curvature, thus avoiding a too high placement, as this could result in biliary reflux and delayed gastric emptying. A jejunal loop 10–15 cm distal to the ligament of Treitz is chosen and placed close to the stomach in an isoperistaltic conformation and not under tension either in an antecolic or a retrocolic fashion [6]. Common complications of the SGE include infections, gastroparesis, hemorrhage, anastomotic leak (mostly between the third to fifth postoperative day), and marginal ulcer (gastrojejunostomy predisposes the part of the jejunum nearest to the stomach to ulcer development because it lacks the protective mechanisms of the duodenum) [6][7][6,7]. Laparoscopic SGE demonstrates improved morbidity and mortality rates, as well as shorter hospitalization, quicker resumption of eating, diminished intraoperative bleeding, and decreased requirements for opiates postoperatively [8]. Surgery is performed on patients with life expectancy of at least several months, a duration deemed ample to navigate and overcome the risk and complications of the surgical intervention [4]. Also, surgery may be performed as a salvage option in selected patients after unsuccessful EUS-GE [9].3. Self-Expandable Metallic Stents
SEMS is the oldest one of the endoscopic techniques used for GOO. SEMSs are available in a condensed form within a device designed to be inserted through the working channel of an endoscope, over a wire that has previously been inserted through the obstruction [4] The stent can then be deployed with or without the use of fluoroscopy. The stent automatically expands to reach its maximum diameter within the following 24 to 48 h. An endoscope with a broad working channel (i.e., =3.7 mm) is required. The majority of cases are managed using therapeutic gastroscopes; however, situations involving enlarged stomachs or strictures in the distal duodenum may be more effectively addressed with a colonoscope or, in some cases, a duodenoscope [10][11][10,11]. There are three types of SEMS: uncovered (uSEMS), partially covered (pcSEMS), and fully covered (fcSEMS). Generally, it appears that both uSEMS and fcSEMS exhibit comparable effectiveness. Studies suggest that all SEMSs have similar technical success (ranging between 89% and 98%) and clinical success, typically characterized as the alleviation of obstructive symptoms and increase in oral consumption [5], ranging between 63% and 93% [12]. The choice of the stent type is based on the individual characteristics of the stenosis, knowing that fcSEMS have higher migration rates, whereas stent ingrowth rate is higher with uSEMS. The latter offer the additional advantage of improved bile outflow (through the stent’s mesh interstices) when the stent is positioned across the papilla in the duodenum [4][5][4,5]. The rate of adverse events (AEs) associated with SEMS placement varies between 0% and 30%, based on the definition applied in each study. This may include minor AEs such as nausea, vomiting, and mild abdominal pain, or major AEs, such as bleeding, perforation, stent migration/displacement, or cholangitis [5]. Delayed AEs are commonly associated with stent dysfunction, resulting from migration or blockage due to food impaction and/or tumor ingrowth/overgrowth. Several methods (e.g., stent clipping or suturing, anti-migratory design) are recommended to diminish the risk of stent migration [13]. The placement of a SEMS is the first-line strategy in patients with short life expectancies (<3 months) and can also be an alternative, if EUS-GE is not successful [9].4. EUS-Guided Gastroenterostomy
EUS-GE is a new endoscopic treatment for GOO offering equivalent efficacy compared to other techniques but with possibly fewer adverse events in experienced hands. During EUS-GE, the stomach and small intestine are fused with the application of a new type of stent, the lumen apposing metallic stent (LAMS), which was introduced in 2012 [14]. LAMS is a double flanged, fully covered stent that provides a stable anastomosis between two adjacent organs (Figure 1). The presence of broad flanges on both ends results in a better anchoring and allows for equal distribution of pressure on the luminal wall, thus decreasing the risk of migration [14]. For this procedure, a linear echoendoscope with forward or oblique viewing is needed [2]. LAMS can be deployed using many methods. They can be divided into two groups, the direct method and the assisted methods: a. the antegrade EUS-GE traditional downstream method, b. the antegrade EUS-GE rendezvous method, c. the retrograde EUS-enterogastrostomy and the EUS balloon occluded GE bypass (EPASS) [2][15][2,15]. The first four are the most frequently used [2][9][2,9]. Here, the rwesearchers describe the basic steps of each procedure [2][15][16][2,15,16]. The selection of the target site depends on the proximity of the specific segment of the small bowel to the gastric wall, as well as the presence of tumor involvement in the third part of the duodenum. When faced with widespread malignant infiltration in the stomach, performing a gastroenterostomy, whether through surgical or endoscopic means, may not be a viable option [2].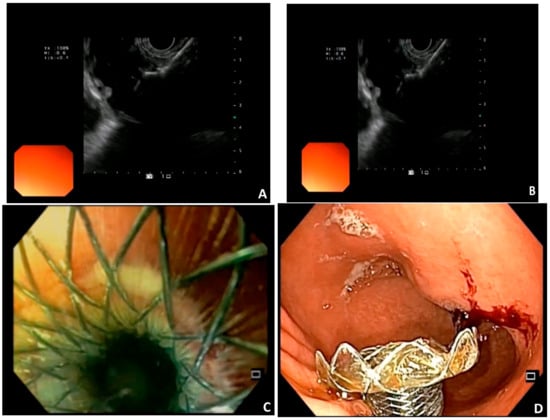
-
Antegrade EUS-GE direct method
- Step 1:
-
Visualize the target intestinal limb by injecting saline or diluted contrast (methylene blue) distal to the obstruction through an orojejunal tube that was previously inserted under endoscopic guidance.
- Step 2:
-
Perform an EUS-guided puncture of the target intestinal limb using a 19-gauge needle.
- Step 3:
-
Aspirate; methylene blue aspiration confirms correct localization of the needle in the target jejunal limb.
- Step 4:
-
Pass either a guidewire through the needle, in order to place a LAMS over it, or use directly a cautery-enhanced LAMS (HOT AXIOS; Boston Scientific Corp.).
-
Antegrade EUS-GE direct method using the wireless endoscopic simplified technique (WEST)
- Step 1:
-
As in Technique 1.
- Step 2:
-
Advance and deploy directly a cautery-enhanced LAMS (HOT AXIOS; Boston Scientific Corp.) in cut mode.
-
Antegrade EUS-GE traditional downstream method
- Step 1:
-
Position a guidewire in the jejunal lumen past the obstruction under endoscopic guidance. Withdraw the endoscope and keep guidewire in place.
- Step 2:
-
Using fluoroscopy advance a dilating balloon over the wire to the jejunum. Dilate the balloon.
- Step 3:
-
From the stomach perform an EUS-guided puncture of the balloon with a 19-gauge needle.
- Step 4:
-
Pass another guidewire through this needle into the jejunum.
- Step 5:
-
Deploy the LAMS over the second guidewire.
-
Antegrade EUS-GE with direct technique over a guidewire (DTOG)
- Step 1:
-
Visualize the target intestinal limb by injecting saline or diluted contrast (methylene blue) distal to the obstruction through an orojejunal tube that was previously inserted under endoscopic guidance.
- Step 2:
-
Perform an EUS-guided puncture of the target intestinal limb using a 19-gauge needle.
- Step 3:
-
Aspirate; methylene blue aspiration confirms correct localization of the needle in the target jejunal limb.
- Step 4:
-
Pass either a guidewire through the needle, in order to place a LAMS over it, or use directly a cautery-enhanced LAMS (HOT AXIOS; Boston Scientific Corp.).
- Step 1:
-
As in Technique 1.
- Step 2:
-
Advance and deploy directly a cautery-enhanced LAMS (HOT AXIOS; Boston Scientific Corp.) in cut mode.
- Step 1:
-
Position a guidewire in the jejunal lumen past the obstruction under endoscopic guidance. Withdraw the endoscope and keep guidewire in place.
- Step 2:
-
Usling fluoroscopy advance a dilating balloon over the wire to the jejunum. Dilate the balloon.
- Step 3:
-
From the stomach perform an EUS-guided puncture of the balloon with a 19-gauge needle.
- Step 4:
-
Pass another guidewire through this needle into the jejunum.
- Step 5:
-
Deploy the LAMS over the second guidewire.
- Step 1:
-
Administer intravenous anticholinergic agent to slow bowel movements
- Step 2:
-
Puncture the target intestinal limb with a 19-gauge needle.
- Step 3:
-
Fill the jejunal limb with contrast medium through the needle and insert a guidewire using fluoroscopy.
- Step 4:
-
Advance the cautery-enhanced LAMS catheter over the guidewire into the jejunal limb in cut mode. Deploy the LAMS.
- 5.
-
Antegrade EUS-GE rendezvous method
- Steps 1 to 3:
-
See Technique 3.
- Step 4:
-
Entrap the puncturing guidewire in the dilating balloon that was punctured, or capture it with an ERCP extraction balloon and/or basket and pull it back outside the mouth, in order to secure it.
- Step 5:
-
Deploy the LAMS using this guidewire under traction.
- 6.
-
Retrograde EUS-EG Enterogastrostomy
- Steps 1 to 4:
-
See Technique 5.
- Step 5:
-
Advance a therapeutic endoscope over the guidewire till you locate the inserted guidewire in the duodenum/jejunum.
- Step 6:
-
Deploy the LAMS in a retrograde fashion by opening the gastric flange first.
- 7.
-
EUS balloon occluded GE Bypass (EPASS)
- Step 1:
-
Using a double-balloon enteroscope (DBE) position a guidewire in the jejunum.
- Step 2:
-
Withdraw the DBE, while keeping the overtube in the antrum or duodenal bulb.
- Step 3:
-
Use a double-balloon-occlusion catheter. This catheter has two balloons (with 20 cm distance between them). Insert it distal to the obstruction under endoscopic control and then inflate both balloons to stabilize the target intestinal limb. Then fill this segment with contrast.
- Step 4:
-
Perform an EUS-guided puncture between the two balloons.
- Step 5:
-
Deploy the LAMS.
