Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Guoying Zhou and Version 2 by Lindsay Dong.
Ischemia reperfusion (I/R) is a common pathological process which occurs mostly in organs like the heart, brain, kidney, and lung. The injury caused by I/R gradually becomes one of the main causes of fatal diseases, which is an urgent clinical problem to be solved. Although great progress has been made in therapeutic methods, including surgical, drug, gene therapy, and transplant therapy for I/R injury, the development of effective methods to cure the injury remains a worldwide challenge.
- ischemia-reperfusion
- exosomes
- signaling pathway
- reperfusion injury
1. Introduction
1.1. Overview of Ischemia-Reperfusion Injury
Ischemia-reperfusion (I/R) injury is a pathological process that can occur in many organs of the human body, accompanied by severe cell damage and inflammatory reactions [1]. In recent years, it has become one of the most common causes of disability and death worldwide [2]. There is a lot of evidence that tissue damage is caused not by ischemia itself, but by the sudden resumption of blood supply after a period of ischemia [3]. Organ ischemia-reperfusion injury often occurs in traumatic shock, surgery, organ transplantation, burns, and other blood circulation disorders [4]. The main organs involved in vascular reperfusion injury are the heart, brain, liver, and kidney, and it can also induce systemic inflammation and eventually lead to multiple organ failure [5]. However, not all organs with ischemia will have I/R injury after blood flow recovery, and many factors can affect its occurrence and severity. For example, if the ischemia time is short, or collateral circulation is easy to form after ischemia, there may be no significant reperfusion injury after the restoration of blood supply. Because oxygen readily accepts electrons and forms oxygen free radicals, organs with a high oxygen demand are more prone to reperfusion injury [6]. In addition, appropriately reducing the temperature and pH value of the perfusion solution and reducing the content of Ca2+ and Na+ in the perfusion solution are conducive to alleviating the reperfusion injury [7]. The pathogenesis of organ I/R injury is summarized in Figure 1.
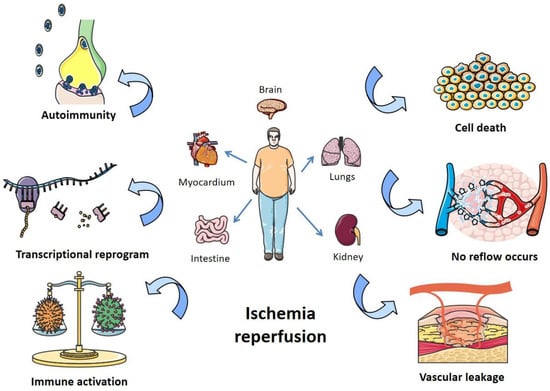
Figure 1. Pathogenesis of organ I/R injury. I/R damages organs like the heart, lungs, kidneys, and intestines, mainly by affecting autoimmunity, transcriptional recoding, immune activation, apoptosis, blocking blood return, and vascular leakage.
1.2. Occurrence and Mechanism of Ischemia-Reperfusion Injury
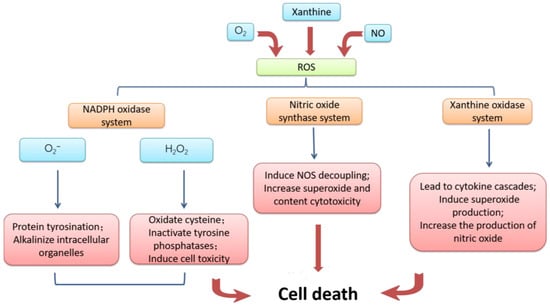
The mechanism of ischemia-reperfusion injury is complex, and the following four aspects are mainly responsible. First of all, the damage to microvessels and parenchymal organs caused by ischemic tissue reperfusion is mainly caused by oxidative stress [8]. Ischemia leads to an inadequate supply of oxygen and nutrients, and cells are unable to produce enough energy. When reperfusion occurs, the sudden supply of oxygen and nutrients increases the degree of oxidative stress within the cell, resulting in the production of excessive reactive oxygen species and other oxidizing substances [9]. The mechanism of cell apoptosis accelerated by massive reactive oxygen is summarized in Figure 2. The synthesis ability of antioxidant enzymes that can scavenge free radicals in ischemic tissue is greatly weakened, and the strong oxidation effect directly damages vascular endothelial cells and tissue cells, thus exacerbating the damage of free radicals to the tissue after I/R [10].

Figure 2. Mechanism of cell damage induced by reactive oxygen species. Excessive accumulation of reactive oxygen caused by I/R and conversion to NADPH oxidase, nitric oxide synthase inhibitor, and xanthine oxidase resulting in cell apoptosis.
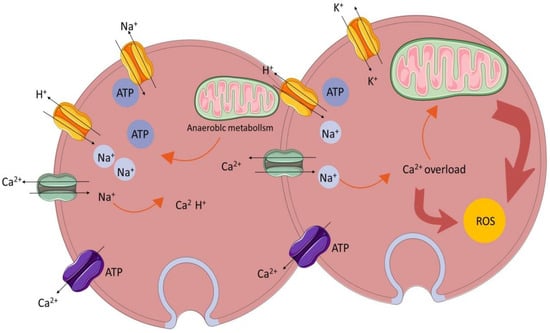
The homeostasis and regulation of the calcium ion concentration in and outside cells are important conditions for maintaining cell function [11]. Calcium overload is the phenomenon of an abnormal intracellular calcium ion concentration leading to structural damage and dysfunction in the functional metabolism [12]. The mechanism of calcium overload is summarized in Figure 3. When I/R injury occurs, the local intracellular calcium ion concentration increases significantly, resulting in the disturbance of calcium ion homeostasis and an insufficient mitochondrial oxidation capacity [13], thus activating phospholipase to damage other organelles or activating calcium-dependent protease activity, as well as increasing the production of oxygen free radicals [14].

Figure 3.
Calcium overload.
In addition, inflammatory responses and immune cell activation also play important roles in the occurrence of ischemia-reperfusion injury [15]. During reperfusion, under the influence of excessive oxidizing substances, immune cells release a large number of inflammatory mediators, causing cell damage in the surrounding tissue [16]. Meanwhile, a large number of free radicals and lysosomal enzymes released by white blood cells make the vascular endothelial cells swell, the microvascular diameter narrow, and permeability increase, meaning that reperfusion blood flow cannot be re-routed [17].
1.3. Current Therapeutic Strategies for Ischemia-Reperfusion Injury
Therefore, reducing inflammation, scavenging free radicals, maintaining calcium homeostasis, and antioxidant therapy are the keys to reducing ischemia-reperfusion injury. In recent years, more and more medical interventions and strategies have been actively applied to minimize I/R injury. However, due to the complex pathologic factors of I/R injury, traditional treatment strategies such as blood pressure control, detumescence, and dehydration are of limited effectiveness [18][19][19,20]. Recently, advanced therapies such as ischemic preconditioning, ischemic post-treatment, antioxidant, hypothermia, and stem cell therapy have emerged as potential treatments for I/R injury [20][21]. Ischemic preconditioning (IPC) is considered as an effective treatment strategy for central nervous system diseases. It causes transient ischemia and the reperfusion of organs or tissues through drugs, intermittent blood flow blockade, and other methods, with the aim of inducing adaptive changes in cells and improving tolerance to subsequent, more severe I/R events [21][22]. Similar to preconditioning, ischemic posttreatment aims to reduce the harmful effects of reperfusion by activating protective signaling pathways and reducing apoptosis [22][23]. Several drugs and compounds have shown promise in reducing I/R injury. These include antioxidants (vitamin C), anti-inflammatory agents (corticosteroids), and mitochondrial protectors (CoQ10), which are used to reduce oxidative stress and maintain mitochondrial function [23][24]. Cooling damaged tissues or organs can slow metabolic processes and reduce oxygen and energy consumption, thus reducing tissue damage. Hypothermia therapy has been shown to be beneficial in reducing I/R injury, especially in the brain and heart [24][25].
Cell therapy is a new approach to the treatment of ischemia-reperfusion injury, and its effects are constantly being studied and explored. Stem cells can promote the healing of I/R injury by repairing tissue cell damage and stimulating the body’s own cell regeneration, secreting vascular growth factors to improve blood supply and releasing apoptosis inhibitors to reduce the occurrence of apoptosis [25][26].
