You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Margarita L. Martinez-Fierro and Version 2 by Jason Zhu.
Fibroblasts synthesize collagen, fibronectin, and extracellular matrix (ECM). Myofibroblasts—other cells involved in fibrosis—secrete factors such as VEGF and TGF-β, produce denser but more disorganized ECM than fibroblasts, and persist longer at the injury site. One cytokine involved in tissue repair is TGF-β. Sources of TGF-β include platelet granules and macrophages. TGF-β is predominantly expressed in PF and helps stimulate the formation of ECM, collagen, fibronectin, elastic fibers, and matrix substances.
- antifibrotic drugs
- pulmonary fibrosis
- COVID-19
1. Cells Involved in the Development of IPF
Several cells contribute to the development of PF by producing growth factors and cytokines, which contribute to the fibrotic process and pathogenesis of the disease.
1.1. Macrophage Activation
Macrophages are a type of cell that plays an essential role in innate immunity and inflammation [1][54]. There are two main macrophage phenotypes: M1, which is associated with proinflammatory responses, and M2 macrophages (M2a, M2b, M2c, and M2d), which play crucial roles in anti-inflammatory processes [2][55]. The transition from one phenotype to another is highly variable, and activation can be a rapid and reversible process [3][56]. Macrophage activation occurs through several pathways. The canonical IRF/STAT signaling pathways are activated by IFN and TLR to activate the M1 macrophage phenotype (STAT1) or to activate the M2 phenotype. IL-4 and IL-3 (STAT6) are required [4][57]. M1 macrophages increase the regulation of IRF5, thereby stimulating cytokines (IL-12, IL-23, TNF) involved in Th1 and Th17 responses [5][58]. On the other hand, it has been seen that interleukin IL-10 can activate the expression of genes mediated by STAT3, which can be associated with the M2 phenotype [6][59].
Two populations of macrophages have been identified in the regulation of pulmonary homeostasis: alveolar macrophages (AMs), which are located in the lumen of the airways, whose markers are CD11blow, CD11c++, and CD169+, and interstitial macrophages (IMs), which are located in the lung parenchyma, with markers CD11b+, CD11clow, CD169 [7][60]. Macrophage signaling pathways in PF are TGF-β/Smad, Wnt/β-catenin, and interleukin signaling [8][9][10][61,62,63]. In a study conducted with patients with severe COVID-19, the accumulation of CD163+ macrophages were reported, some of which co-expressed the chemokine receptor CXCR3 and complement factor C1Q. In addition, collagen deposition was reported very prominently, for which the authors propose that SARS-CoV-2 promotes genetic programs associated with fibrosis in macrophages [11][64].
1.2. Activation of Fibroblasts and its Differentiation to Myofibroblasts
Fibroblasts are mesenchymal cells whose function is homeostasis and production of ECM [12][65]. In the lung, fibroblasts are found in the ECM of the interstitial space, where they participate in early and late wound repair [13][66]. For the progression of PF, a key factor is the proliferation and differentiation of fibroblasts into myofibroblasts [14][67]. TGF-β 1 participates in stimulating the differentiation of fibroblasts into myofibroblasts [15][68]. A study found that miR-21 expression is involved in collagen production by fibroblasts or fibroblast-like cells through negative regulation of Smad7. This suggests that Smad7 could be a potential therapeutic target in PF [16][69].
In IPF, type 1 collagen is the most abundant and relevant collagen, as it is deposited in greater quantities in the ECM [17][18][70,71]. Fibroblasts and myofibroblasts mainly secrete this type of collagen due to the action of several cytokines and factors, such as TGF-β, in addition to the hypoxia pathway [19][72]. In a study conducted by Philp, C. J et al., the inhibition of the formation of crosslinks through transglutaminase 2 modified the behavior of fibroblasts by reducing adhesion and proliferation of these; it also improved the renewal of ECM, which would mean that it could be a potential therapy for IPF by reducing excess ECM at the site of injury [20][73]. Figure 1 displays antifibrotic drugs with evidence of their use in PF related to COVID-19 and their possible mechanisms of antifibrotic action. For instance, Nimotuzumab inhibits EGFR, thereby inhibiting the JAK/STAT pathway and preventing fibrosis from progressing or occurring [21][74].
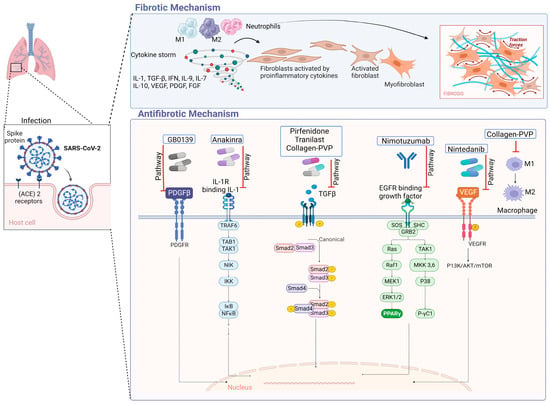
Figure 1. Antifibrotic drugs. Figure shows a possible antifibrotic mechanism involved in the treatment of pulmonary fibrosis induced by COVID-19. Pirfenidone, Nintedanib, and collagen-polyvinylpyrrolidone have different mechanisms that may regulate PF, while Nimotuzumab, Anakinra, Tranilast, and GB0139 have more specific mechanisms of action [22][23][24][25][26][27][28][75,76,77,78,79,80,81].
2. Signaling Pathways Involved in the Development of IPF
The pathological process by which IPF develops is very complex and involves the activation of alveolar epithelial cells as well as the proliferation of fibroblasts and differentiation into myofibroblasts; likewise, various growth factors such as TGF-β intervene, leading to irreversible damage to the alveolar architecture.
2.1. TGF-β Signaling
TGF-β is a member of a family of polypeptides that modulate various cellular functions, the most important of which are cell proliferation, differentiation, and apoptosis [29][82]. There are three major inactive isoforms of TGF-β (TGF-β1, TGF-β2, TGF-β3), which are synthesized and secreted in association with latency-associated peptide (LAP) [30][83]. When there are normal conditions, TGF-β in its inactive conformation binds to LAP, which, through disulfide bonds, binds to latent TGF-β-binding proteins, crosslinked with the ECM [31][84]. TGF-β can be activated within the large latent complex (CLL) in various ways, for example, acidification, temperature variations, oxidation, proteolytic cleavage, or through interaction with integrins, which will cause TGF-β to interact with TGF-β receptors (TGF-βR), and the consequent action is to mediate downstream effects [32][85]. TGF-β plays an important role in the development of IPF [33][34][86,87]. Upregulation and activation of all TGF-β isoforms and receptors have been reported to be involved in the pathogenesis of IPF [35][88].
It is postulated that TGF-β signaling, which is a profibrotic inducer, is stimulated by the protein N of SARS-CoV. Since this protein is 90% similar to that of SARS-CoV-2, it is considered to be one of the possible mechanisms of pulmonary fibrosis associated with SARS-CoV-2 infection [36][89]. Another possible mechanism is that coronaviruses can induce the lowering of ACE2; therefore, there is a reduction of angiotensin II (Ang II) in the lungs. In this way, Ang II could upregulate TGF-β and connective tissue growth factor [37][47]. Once the lung injury occurs, TGF-β, in addition to being released by the injured epithelial and endothelial cells, is released by macrophages, fibroblasts, T cells, and monocytes, which results in a self-sustained process [32][85]. The canonical TGF-β signaling pathway will involve the activation of SMAD proteins that will activate transcription [38][90]. TGF-β, in its non-canonical pathway, can act on mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and N-terminal Jun N kinase (JNK), as well as RHO-associated kinase (ROCK) and AKT pathways [39][91]. Some of these pathways are involved in the induction of the epithelial–mesenchymal transition (EMT), which promotes the exacerbation of fibrotic lesions [40][92].
2.2. Activation of Integrins
Integrins belong to a family of proteins composed of one of 18 unique α subunits and eight β subunits that pair to create one of the 24 identified integrins [41][93]. They play a crucial role in maintaining cell–cell junctions, cell–EM adhesion, and signal transduction from the ECM to the intracellular compartment [42][94]. Integrins have been found to participate in various disease processes, including sarcoidosis and PF [43][95]. Recent research has demonstrated that the S protein of SARS-CoV-2 binds to integrin ⍺5β1 in vascular endothelial cells (ECs) through the RGD domain. This binding activates the expression of nuclear factor kappa β (NF-κβ), which is responsible for vascular leakage and leukocyte adhesion. These findings suggest a new mechanism by which SARS-CoV-2 affects ECs and identify integrin ⍺5β1 as a potential therapeutic target for treating COVID-19-related inflammation [44][96]. αVβ6 is another integrin that recognizes RGD (Arg-Gly-Asp). It is overexpressed in many aggressive epithelial tumors, as well as in pulmonary and hepatic fibrosis [45][97]. A study found that high levels of αVβ6 suggest the presence of fibroblast foci and can also identify a specific endotype of progressive fibrotic lung disease [46][98].
3. Growth Factors Involved in Idiopathic Pulmonary Fibrosis
Several growth factors, including PDGF, CTGF, and FGF, have been reported to play a role in the development of IPF by regulating the activity of fibroblasts.
- -
-
PDGF is a mitogenic molecule that affects various connective tissue cells and other cell types [47][99]. This factor is composed of polypeptide chains A and B linked by a disulfide bond, which can form homo- and heterodimers [48][100]. PDGF is produced by several cell types, including macrophages, platelets, endothelial cells, and fibroblasts [49][101]. It has four isoforms that bind to two receptor tyrosine kinases (PDGFR α and β). These receptors are expressed in higher quantities in cells such as fibroblasts and myofibroblasts, where they participate in survival, proliferation, and migration [50][102]. PDGF works together with TGF-β to promote the release of activated alveolar macrophages and epithelial cells, which are directly involved in the self-activation cycle of fibrosis [51][103]. High levels of PDGF have been associated with FP, both in lung tissue and in bronchoalveolar lavage fluid, as demonstrated in various animal studies with models of PF [52][104]. PDGF-A and PDGF-C have also been found to be overexpressed in various cell types in a mouse model of injured lungs [53][105]. During pulmonary fibrosis, alveolar macrophages promote the release of PDGF, which contributes to the proliferation of alveolar myofibroblasts and fibrogenesis [54][106].
- -
-
Fibroblast growth factors (FGFs) belong to a family of 22 members [55][56][107,108]. These can be divided into hormone-like FGFs (FGF 15/19, 21 and 23), canonical FGFs (FGF 1–10, 16–18, 20 and 22), and intracellular FGFs (FGF 11–14) [57][109]. FGFs are involved in several biological responses, interacting with heparan sulfate glycosaminoglycans (HPSGs). Released FGFs interact with cell surface receptor (FGFR) domains [58][110]; a complex of FGF, FGFR, and HPSG is formed, and thus, signaling is carried out [59][111]. Abnormal FGF signaling is implicated in various pathologies and is known to participate in the pathogenesis of PF [60][112]. The inhibition of FGF signaling by using FGFR2c decreased lung fibroblast proliferation and differentiation in vitro and in vivo in a murine model [61][113]. The alteration in FGFR1 and FGF1 signaling is critical for fibroblast migration in PF [62][114]. In SARS-CoV infection, the EGFR signaling pathway remains active even after the virus is eliminated. This pathway is believed to be responsible for the effect of FGF in the conduction to PF [63][115].
- -
-
Connective tissue growth factor (CTGF) belongs to the IGFBP family, which is known as insulin-like growth factor-binding protein 8 (IGFBP-8) [64][65][116,117]. This 38 kDa mitogenic peptide is involved in fibrotic processes and stimulates migration, fibroblast proliferation, and ECM production [66][118]. CTGF is a cytokine that participates in the activation of fibroblasts; it was observed that in alveolar epithelial cells, the expression levels of CTGF were drastically reduced by inhibiting the Rho pathway. Additionally, the epithelial cells of the lung in the mice indicators of CTGF increased the activity of the promoter of CTGF when the authors treated them with bleomycin, an inducer of PF [67][119]. CTGF can interact with other molecules to develop its fibrotic effects, thus contributing to the generation of fibrosis; among the molecules with which it interacts, there are cytokines and growth factors such as IGF1, BMP4, BMP7, TGF-β and VEGF. This interaction with other molecules may positively or negatively alter the signaling pathways of which they are participants, resulting in changes in cell adhesion, migration, differentiation, angiogenesis, myofibroblast activation, deposition, and remodeling of ECM, which causes changes in structure and alters tissue remodeling [68][120].
- -
-
Metalloproteinases (MMPs) belong to the family of extracellular endopeptidases, whose primary function is the degradation of ECM components, while tissue inhibitors of metalloproteinases (TIMPs) block the activity of MMPs [69][121]. The participation of proinflammatory cytokines affects the overexpression of MMPs, which increases its activity and thus participates in the remodeling of the airways [70][122]. The level of TIMPs is increased in the presence of a fibrotic process such as PF [71][123]. MMP-2 and MMP-9 are downregulated in severe COVID-19 patients [72][124]. MMP-2 has shown a correlation with mortality in patients with COVID-19, so it could be a potential prognostic predictor for patients with COVID-19 [73][125]. One study reported that MMP-7 correlates with clinical and functional predictors of disease severity and mortality, so it can be used to distinguish IPF from other chronic diseases [74][126].
