Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Manmohan Singhal and Version 2 by Catherine Yang.
Neurological ailments, including stroke, Alzheimer’s disease (AD), epilepsy, Parkinson’s disease (PD), and other related diseases, have affected around 1 billion people globally to date. PD stands second among the common neurodegenerative diseases caused as a result of dopaminergic neuron loss in the midbrain’s substantia nigra regions. Medicinal plants, herbal formulations, and natural bioactive molecules have been gaining much more attention in recent years as synthetic molecules orchestrate a number of undesired effects. Several in vitro, in vivo, and in silico studies in the recent past have demonstrated the therapeutic potential of medicinal plants, herbal formulations, and plant-based bioactives.
- phytocompounds
- Parkinson’s disease
- phenolic acids
- antiparkinsonian
- neuroprotection
1. Introduction
The brain is the most complicated organ of the body consisting of a complex network of neurons, and functions as a site of intelligence, memory, and cognition, the initiator of body movement, the interpreter of the senses, and the manager of behaviors. It mainly consists of billions of nerves, which are in regular communication through trillions of connections called synapses [1][2][1,2]. The brain is subjected to various forms of stresses, including oxidative stress (OS) resulting from the body’s oxygen requirements/utilization and high content of unsaturated fatty acids [3][4][3,4]. Nerve cells in the mid-brain degrade slowly, leading to movement- and coordination-related problems, ultimately resulting in neuropathophysiology. Neurodegeneration is associated with the progressive damage of neuronal tissue that causes the irrecoverable loss of neuronal function, subsequent decline in cognitive function, and motor activity [5][6][7][5,6,7]. Among the neurodegenerative diseases, Parkinson’s disease (PD), mild cognitive impairment (MCI), Alzheimer’s disease (AD), epilepsy, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD) are of primary importance with distinguished pathological etiologies and clinical management [8][9][8,9].
PD is the second common chronic neurodegenerative disorder after AD seen mostly in the aging population [10][11][10,11]. It is characterized by synucleipathy, wherein neurons in specific part of the brain undergo damage, resulting in motor signs of muscle stiffness, tremor, and postural instability. Additionally, synucleipathy more specifically refers to a class of neurodegenerative disorders that are characterized by a prion-like spread through interconnected neuronal networks and the abnormal aggregation of α-syn inside glial or neuronal cells. However, the complete etiologies of PD still remain unclear [12][13][14][12,13,14]. The disease’s progression has been pointed out as being related to the depletion of dopamine in the nigrostriatal pathway. Further, intracytoplasmic inclusions of dopamine called Lewy bodies have been identified in patients with PD [12][13][12,13]. The deterioration of dopamine-producing neurons inside the substantia nigra (SN), with reduced dopamine release in the striatum, is a major cause of the disease. The SN is located in the midbrain, posterior to the crus cerebri fibers of the cerebral peduncle. It consists of two regions: the substantia nigra pars compacta (SNpc), which harbors dopaminergic neurons, and the substantia nigra pars reticulata (SNpr), comprising gamma-aminobutyric acid-responsive (or GABAergic) neuronal cells. The putamen, striatum, and caudate nuclei are well connected with dopaminergic projections from the SNpc. Each side of an adult SNpc has 400–500 thousand dopaminergic cells, constituting a negligible fraction of total brain mass. As evident in PD, these tiny clusters of cells have a disproportionate impact on motor output and behavior. When compared to other neurons, dopaminergic neurons in the SNpc are more vulnerable to oxidative stress. In the ventral midbrain, these neurons extend to the striatum and play a crucial role in regulating motor behavior in mammals. The SNpc comprises a cluster of cells that releases the neurotransmitter dopamine in the striatum connected to the basal ganglia. The basal ganglion in turn is linked to the thalamus and motor cortex, which are associated with controlling motor output. PD is predominantly attributed to the loss of the majority of cells in the SNpc region [15][16][17][15,16,17]. The factors that are involved in the progression of PD includes ROS, neuroinflammation, mitochondrial dysfunction, the misfolding of proteins, and protein agglomeration, along with several other environmental factors and biological processes [11].
2. Medicinal Plants, Herbal Formulations and Plant-Based Bioactives (Polyphenols, Terpenes, and Alkaloids) as a Potent Therapeutics for PD Management
Medicinal plants possessing high concentrations of additional nutritional elements provide both health advantages and increased nutritional value due to their potency to influence metabolic processes. In the recent decade, medicinal plants and bioactive constituents with diverse structures have shown to be promising resources for PD drug research.2.1. Polyphenolic Compounds
Polyphenols are a class of significant natural bioactive molecule has been found to be broadly distributed in dietary vegetation and display potential neuroprotective properties against neuroinflammation and neuronal death as evidenced from both in vitro and in vivo experimentation. They have been further classified as flavonoids and non-flavonoids consisting of bioactive compounds including stilbenes, lignans, phenolic acids, curcuminoids, and coumarins. Polyphenols are secondary metabolites synthesized in plants by the polyketide or shikimate pathway and commonly found in vegetables, fruits, nuts, and seeds. They possess multiple phenol units (C6H5OH) with hydroxyl groups (OH) linked to the aromatic benzene ring. Polyphenols have been found to exhibit potential therapeutic properties. A polyphenols-rich diet has demonstrated significant modulatory effects on the pathophysiological mechanisms of many underlying chronic diseases, especially in diabetes and cardiovascular and neurodegenerative diseases as evidenced from several experimental and clinical studies, indicating their significant prophylactic and therapeutic potential [18][82]. A growing body of evidence has demonstrated that the supplementation of polyphenolic compounds limits the risk for neurodegenerative disorders. Several of these compounds have been found to have cell-protective abilities against oxysterols (e.g., 7-ketocholesterol),mitigate mitochondrial dysfunction and cell injury. Polyphenols, namely resveratrol, quercetin, and apigenin, have shown potential scavenging activity against reactive oxygen species (ROS) induced by oxysterols and thereby counteracting ROS [19][83]. They possess influential antioxidant properties owing to their free radical scavenging potential and iron chelating action. Additionally, these have been further documented to display antiviral, antibacterial, anti-inflammatory, anticarcinogenic, and neuroprotective properties [20][84]. Recently, it has been established that plant polyphenols orchestrate neuroprotective abilities such as the capacity to combat misfolded protein gathering, the probability to endorse cognition, memory, ROS, neuroinflammation, neurotrophin secretion, and the capability to shield nerve cells after neurotoxins exposure [21][85]. These compounds possess at least one OH group existing over the aromatic side chain with its backbone having a simple moiety to a multifaceted polymer [22][86].-
Structure activity relationship
-
In vitro and in vivo studies
2.1.1. Flavonoids
Flavonoids have a common 1,2-diphenylpropane or 1,3-diphenylpropane (C6-C3-C6) basic structure [35][99]. Various biological properties of flavonoids include antithrombotic, anticancer, anti-inflammatory, antimicrobial, antiviral, and immunomodulation. Flavonoids have been reported in a wide variety of vegetables (tomatoes, onion, cabbage, cauliflower) and fruits (apple, grapes, berries, banana). Li et al. have revealed that these compounds are beneficial to skeletal muscles, liver, pancreas, adipocytes, and neuronal cells [36][100]. The results of the randomized clinical trials have demonstrated the enhancement of cognitive abilities resulting from the dietary intake of flavonoids-rich foods, irrespective of age and health status [37][21]. According to structure activity relationship studies, it has been documented that the double bond between the second and third carbon atoms, the 3′,4′-catechol, the ketone group, and the hydroxyl group at the third position present in the flavonoid backbone are responsible for the free radical scavenging and antioxidant properties of flavonoids. Because the double bond between C2–C3 is conjugated to the carbonyl group present in the C ring, unsaturated flavonoids have a higher capacity to scavenge free radicals in relation to saturated compounds like flavanones [38][39][101,102]. Mittal et al. mentioned that the extract of Ginkgo biloba rich in flavonoids orchestrates a protective impact on dopaminergic neurons in animal models of PD [37][21]. In vitro and in vivo findings suggest that flavonoids intake (with supplements or with normal diet) could be a promising intervention for the attenuation and/or prevention of the deterioration effects of cognitive decline associated with various neuronal disorders [40][22]. Flavonoids consists of potent bioactive compounds including genistein, baicalein, epigallocatechin-3-gallate (EGCG), and hesperidin (Figure 13).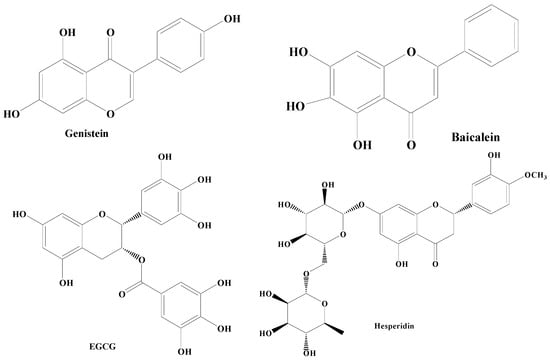
Figure 13.
Different types of flavonoids.
2.1.2. Non-Flavonoids
Non-flavonoids are also polyphenolic compounds exhibiting various types of neuroprotective effects in PD by different mechanisms of action. This group of compounds comprises phenolic acids, phenolic alcohols, stilbenes, and curcumin.Phenolic Acids
Phenolic acids are secondary metabolites produced by plants. They exist as acidic complexes in the form of hydroxycinnamic and hydroxybenzoic acids (Figure 24A,B) [67][129]. They possess important biological and pharmacological properties including anti-inflammatory, anticarcinogenic, anticancer, antioxidant, and antimutagenic. Due to the presence of the phenol group and resonance-stabilized conformation, phenolic acids stand out among other chemicals of natural origin for their potent antioxidant and anti-inflammatory activity, which is achieved by a radical scavenging mechanism. Studies carried out using PC12 cells have demonstrated that phenolic acids mitigate 1-methyl-4-phenylpyridinium (MPP+)-induced cell death by boosting the neurite network and triggering the production of proteins essential for synaptogenesis (synaptophysin and synapsin I) and axonal growth (GAP-43). Findings of studies have also demonstrated that phenolic acid reduces the sickness behavior and neuroinflammation induced by lipopolysaccharide (LPS) in mice. TNF-α, a measure of inflammation, has been found to be decreased in the serum in a dose-dependent way, when phenolic acids were administered orally (30 mg/kg) one hour before LPS exposure (1.5 mg/kg) [68][130]. Phenolic acids are composed of a wide array of bioactive compounds including gallic acid, coumaric acid, ellagic acid (EA), salvianic acid, and rosmarinic acid.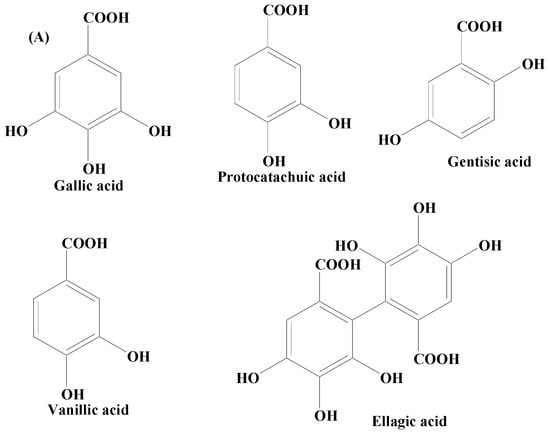
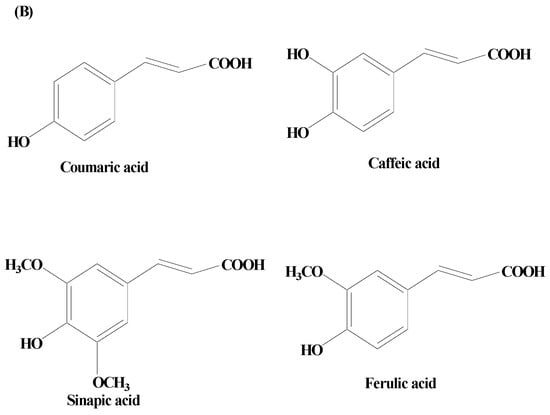
Figure 24.
(
A
) Phenolic acids—hydroxycinnamic acid. (
B
) Phenolic acids—hydroxybenzoic acid.
Phenolic Alcohols
Phenolic alcohols reported in various plants are also polyphenolic compounds containing an OH group linked to an aromatic hydrocarbon. Plants synthesize this class of compounds in order to combat environmental stresses including pathogens or insects that attack plants [88][89][150,151]. Phenolic alcohols orchestrate antioxidant and anti-inflammatory activities by rescuing nerve terminals in the striatum and dopaminergic neurons in the SNpc area and restoring SOD, CAT, and glutamate levels, preventing lipid oxidation, reducing the level of ionized calcium binding adaptor molecule (Iba-1), GFAP hyperactivity, pro-inflammatory cytokines, iNOS, and COX-2 activities, respectively. They contain an OH moiety linked to an aromatic hydrocarbon which helps to scavenge free radicals and protect the neuronal damage. In vitro and in vivo studies have shown that phenolic alcohols have significant levels of anti-inflammatory and antioxidant properties due to which they play a very important role in managing PD symptoms [90][152]. The major phenolic alcohols found predominantly in plants are 6-Shogaol and sesamol. 6-Shogaol (6S) is a pungent ingredient extracted from ginger. It exhibits a wide range of pharmacological properties including neuroprotective potential by overpowering neuroinflammatory factors including TNF-α, NO, COX-2, and iNOS. In addition to these, it also acts through the activation of microglia in the SNpc. Several investigations have documented its neuropharmacological effects for neurodegenerative disorders. In an AD transgenic mice model, 6S prevented aberrant buildup of the Aβ-peptide in the hippocampus and cortical areas and improved memory impairment. Additionally, it has been documented to reduce memory loss, neuronal damage, and neuroinflammatory effects in mice. In studies on 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine (MPTP)-induced PD models, the anti-PD effects of 6S have been investigated. These studies revealed that 6S remarkably prevents dopaminergic neuronal damage, MPTP-induced motor impairment, and striatal dopamine depletion. In MPTP-induced PD mice, 6S prevents gliosis, dopaminergic neuronal degeneration, and motor impairment. Further, it prevents nuclear factor B’s increased nuclear translocation, as well as apoptotic cell death [91][92][93][94][153,154,155,156]. Sesamol, a lignin obtained from the shrub Sesamum indicum, holds a wide range of defined neuroprotective potential, and remains utilized as therapeutics against PD. Owing to high metabolic activity, brain requires a lot of energy inputs for physiological functions. The brain also features a high membrane surface to cytoplasm ratio, a low repair capability, a non-replicating nature of neurons, and a comparatively low antioxidant machinery. Due to an imbalance between pro-oxidant and antioxidant agents in the brain, increased free radicals, which are mostly created through oxidative phosphorylation, play a significant role in neurological illnesses. This supports the need to target antioxidant systems in order to combat OS and the resulting brain disorders. By encouraging antioxidative defense systems for neutralizing free radicals and by limiting transcription, the antioxidant system is crucial for saving neuronal cells from OS and maintaining the proper redox balance in the brain tissue. Sesamol has been found to boost the action of various antioxidant enzymes, namely glutathione reductase, CAT, SOD, GPx, and also non-enzymatic antioxidants (vitamin E, GSH, and vitamin C), thereby reducing the levels of the lipid peroxidation and nitrites [95][96][97][98][157,158,159,160].Stilbenes
Stilbenes are polyphenolic compounds found in various plant species. They have been shown to have anti-inflammatory properties, estrogen receptor agonist qualities, and effects on cell proliferation, cell signaling pathways, and apoptosis. Stilbenes also exhibit antifungal, antiviral, and antibacterial properties. They possess numerous beneficial attributes for the inhibition of abundant pathophysiological issues, namely age-linked ailments (example: type 2 diabetes mellitus, and obesity), OS, and neurodegenerative disorders including PD [99][100][161,162]. In addition, an oligostilbene compound, Amurensin G, found in Vitis amurensis (a type of wild grape) root was revealed to maintain the survivability of SH-SY5Y cells by downregulating α-syn and ubiquitinated proteins [101][102][163,164]. A stilbene compound, resveratrol, occurring naturally in several plants including Polygonum cuspidatum has demonstrated protective potential in the animal and cellular models of PD (Figure 35). Heme oxygenase-1 (HOX-1) is a 32 kDa stress response protein implicated in the prevention of PD. HOX-1 functions in response to stress and eventually becomes downregulated. Resveratrol exhibits neuroprotection against paraquat-induced PC12 cells via HOX upregulation. Supplementation of resveratrol has been found to upregulate the SOD enzyme activity [103][165]. The in vitro findings on antiparkinsonian activity of this compound have revealed the capability of resveratrol to downregulate the level of caspase-3 enzyme activity and the lactate dehydrogenase (LDH) leakage. On a similar line, its hydroxylated derivative oxy-resveratrol also has reported neuroprotective potential through the decrease in ROS generation inside the cell, reduction in phospho-JNK-1 and 2, and cytosolic SIRT1. Due to its hydroxyl group and competing with coenzyme Q, resveratrol decreases the activity of complex III, hence lowering the formation of ROS. Resveratrol has also been demonstrated to protect oxygen glucose deprivation and reperfusion (OGD/R), at least in part, according to a study on the PC12 cell line. It exerts neuroprotective effects by reducing OGD/R-induced OS and maintaining mitochondrial function through PINK1/Parkin-mediated mitophagy [41][103]. Studies carried out using two-month-old male rats with middle cerebral artery occlusion (MCAO) treated with rehabilitation training and resveratrol indicated that resveratrol enhanced neurological and motor function in MCAO rats via activating the brain-derived neurotrophic factor/tyrosine kinase receptor B (BDNF/TrkB) signaling pathways and SIRT1 signaling network. In both in vivo and in vitro experimental models of neurodegeneration, studies have shown that resveratrol moderates mitochondrial activity, maintains redox homeostasis, and cellular dynamics [104][166].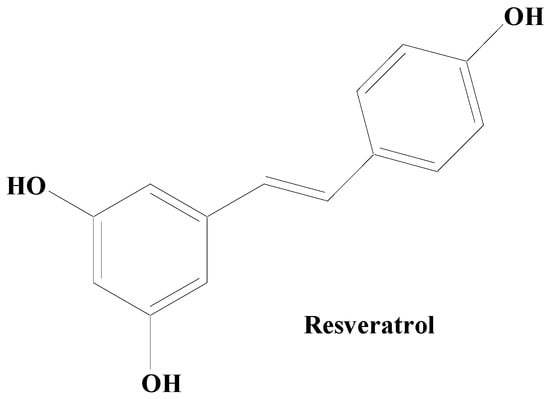
Figure 35.
Natural stilbene—resveratrol.
Curcumin
Curcumin, also known as diferuloylmethane is a polyphenolic compound obtained from the plant rhizome Curcuma longa. Curcumin exhibits, a broad array of pharmacological properties owing to its hydroxy (antioxidant activity) and methoxy (antitumor and anti-inflammatory activities) groups (Figure 46). It has been established to possess various health benefits. Curcumin’s therapeutic and prophylactic efficacy has been demonstrated in many neurodegenerative, oncological, inflammatory, and autoimmune diseases. Studies carried out on an antiparkinsonian rat model revealed that curcumin treatment potentially controls the PD complications via the dopaminergic neuronal damage and depletion of DA. Moreover, iron chelating activity in addition to condensed iron-positive cells in SN has also been mitigated by curcumin. Research findings have shown that curcumin has the ability to downregulate the level of the caspase-3 enzyme, besides amplifying the LRRK2 mRNA and protein expression in vitro. Accumulated evidence showed that curcumin exhibits various neuroprotective properties, including chelating metal ions’ antioxidation, inhibition of the aggregation of misfolded proteins, and attenuating neuroinflammation [105][106][167,168]. According to Jin et al., curcumin was found to alleviate PD by energizing the BDNF/PI3k/Akt transduction pathway [107][169]. Additionally, a nanoformulation of this medication plus levodopa was recently suggested for the treatment of PD [108][170]. In a PD model, curcumin also offers protective effects on the cerebellum [109][171]. Earlier biochemical findings established that curcumin proficiently blocked aggregation of α-syn in vitro. Various improved equivalents of curcumin having enhanced constancy have also been confirmed to be effective in preventing depolymerizing α-syn fibrils and α-syn amyloid aggregation. In vivo studies have demonstrated that curcumin has no effect on how α-syn condensates develop or their initial shape. It does however effectively prevent α-syn from amyloid genesis by reducing its fluidity within the condensates. Additionally, it prevents α-syn E46K and H50Q mutants that are linked to PD illness from aggregating amyloid under phase separation. Curcumin can also weaken α-syn amyloid aggregates that have already developed in the condensates [105][106][167,168].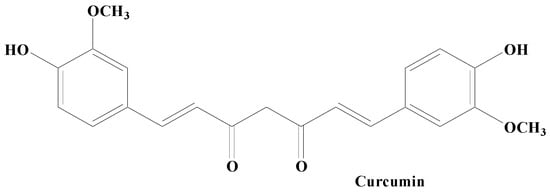
Figure 46.
Curcumin.
2.2. Terpenes
Terpenes are one among the most widely distributed compounds in the plant kingdom. They possess the utmost molecular dissimilarity amongst the secondary metabolites. Terpenes are mainly obtained from coniferous plants, namely juniperus, abies, pinus, and picea. They are mainly hydrocarbons constituting the main bioactive components of natural products including essential oil, wax, rubber, and resin. Terpenoids have biological and pharmacological potential including anticancer, antiviral, anti-inflammatory, antifungal, antihyperglycemic, antiparasitic, and antimicrobial [110][111][112][113][172,173,174,175]. Some of the important terpenes includes carnosic acid, ginkgolide B, and celastrol (Figure 57).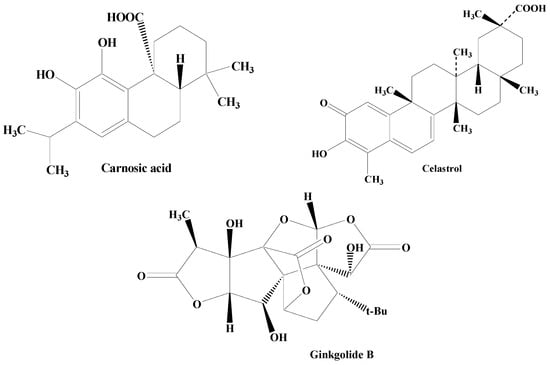
Figure 57.
Structures of various terpenes.
-
Structure Activity Relationship
-
In vitro and in vivo studies
2.3. Alkaloids
Alkaloids are secondary metabolites, consisting of nitrogen, which at the beginning have been considered as the principal group of bioactive natural compounds isolated from plants (Figure 68). Additionally, alkaloids exhibit much structural diversity including in the chemical skeleton such as tetra-hydro-isoquinoline, indole, pyrrolizidine, tropane, piperidine, quinolizidine, indolizidine, pyridine, pyridinone, quinoline, quinazoline, xanthine, steroid, terpenoid, chromone, and flavoalkaloids, respectively [133][195]. A wide variety of biological roles such as antidepressant, emetic, diuretic, antimicrobial, antiviral, antihypertensive, anti-inflammatory, antitumor, anticholinergic, myorelaxant, hypoalgesia, and sympathomimetic have been displayed by alkaloids [134][196]. Various studies have shown that numerous alkaloid components possess a promising relaxing ability for a diverse neuron-related disorders including PD [135][197]. Thus, natural product-based alkaloids having polypharmacology variation characteristics are very beneficial in the progress of drug development in managing PD [136][137][138][198,199,200]. Zingerone and acetylcorynoline are among the important alkaloids possessing diverse pharmacological functions.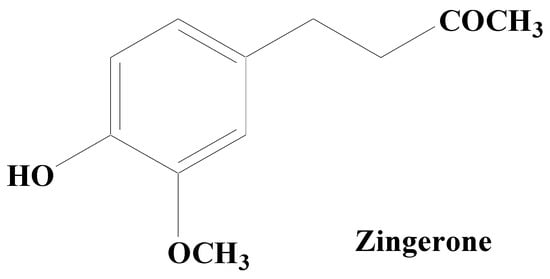
Figure 68.
Zingerone.
-
Structure Activity relationship
-
In vitro and in vivo studies
