Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Neil Lin.
Three-dimensional (3D) printing is a process in which materials are added together in a layer-by-layer manner to construct customized products. Many different techniques of 3D printing exist, which vary in materials used, cost, advantages, and drawbacks. Medicine is increasingly benefiting from this transformative technology, and the field of ophthalmology is no exception. The possible 3D printing applications in eyecare are vast and have been explored in the literature, such as 3D-printed ocular prosthetics, orbital implants, educational and anatomical models, as well as surgical planning and training.
- 3D printing
- bioprinting
- ophthalmology
- prosthetics
- implants
- anatomy
- surgery
- tissue engineering
1. Introduction
Three-dimensional (3D) printing has revolutionized hardware manufacturing as a tool for rapid prototyping and product development. As a form of additive manufacturing, 3D printing fabricates custom constructs layer by layer based on models produced in computer-aided design (CAD) software. In the medical context, images obtained from computed tomography (CT), magnetic resonance imaging (MRI), and 3D scanning may be used to generate patient-specific designs and devices. Key advantages of 3D printers for medical use include the ability to produce complex geometries and the flexibility of using one printer to tailor many diverse designs [1]. These attributes make 3D printing suitable for personalized medicine as printers can cost-effectively produce specialized patient-specific products compared to conventional techniques [2]. Three-dimensional printing has already been established in many medical domains, where it is used to manufacture products from guides for dental surgery to medical instruments to custom orthopedic implants [3,4][3][4].
The potential of 3D printing for precision medicine is further illustrated by its current and potential applications in ophthalmology. Notably, the advancement of printing technologies to enable printing on the nanometer-micrometer scale has increased the utility of 3D printing for ophthalmic devices requiring fine detail, such as intraocular implants, ocular prosthetics, and surgical devices. For medical education, 3D-printed anatomical models can express the subtle anatomical details of the eye to better train students and physicians [5]. Similarly, precision surgical treatment, enabled by 3D-printed surgical guides, can help ophthalmologists reduce operative time and enhance patient outcomes [6,7,8][6][7][8]. Finally, the emerging field of bioprinting is opening avenues for regenerative medicine in ophthalmology with novel research in manufacturing artificial corneal, retinal, and conjunctival tissue models that could yield sight-restoring treatments in the future [9,10][9][10].
2. Build Instructions for 3D Printing
-
Production of a computer-aided design (CAD) model: Each 3D printing project begins with a digital model of the intended product. This model is typically created using computer-aided design (CAD) software or by generating a 3D representation based on CT or MRI scans of an existing object. The geometric data of the 3D model can be stored in a standardized .STL or .OBJ file format (Figure 1).
-
Slicing and preparation of print file: Slicing software is then utilized to divide the 3D model into thin horizontal cross-sections, creating a digital representation of each layer to be printed. This step enables the specification of layer height, infill density, and print speed. If the design contains layers that overhang previous layers, it may require the use of support structures, which can be inserted by slicing software to maintain structural integrity against gravity.
-
Toolpath Generation and Output: Based on the shape of successive layers, slicing software will generate toolpaths for the 3D printer that contain ordered coordinate directions to create the surface geometry, interior fill pattern, and support structures. Toolpaths are converted into control code specific to the 3D printer that guides the printing process.
-
Material Selection and Machine Setup: The printer will be loaded with material and prepared for the physical build process. With the material diversity afforded by 3D printing, the appropriate material should be selected for the desired functional properties of the product.
-
3D Printing Process: The printer will then work layer by layer to additively manufacture the product. The 3D printer largely automates this step, and many have integrated control units that monitor the printing process and alert the user if an issue requiring intervention occurs. Once printing is completed, further steps may include product separation from the build platform and safe handling precautions.
-
Postprocessing: After printing, the 3D model may require postprocessing, such as polishing, further curing, chemical treatment, coloring, and the removal of support structures, depending on the target function and appearance requirements. Support structures may be removed manually or via dissolution with a targeted solvent.
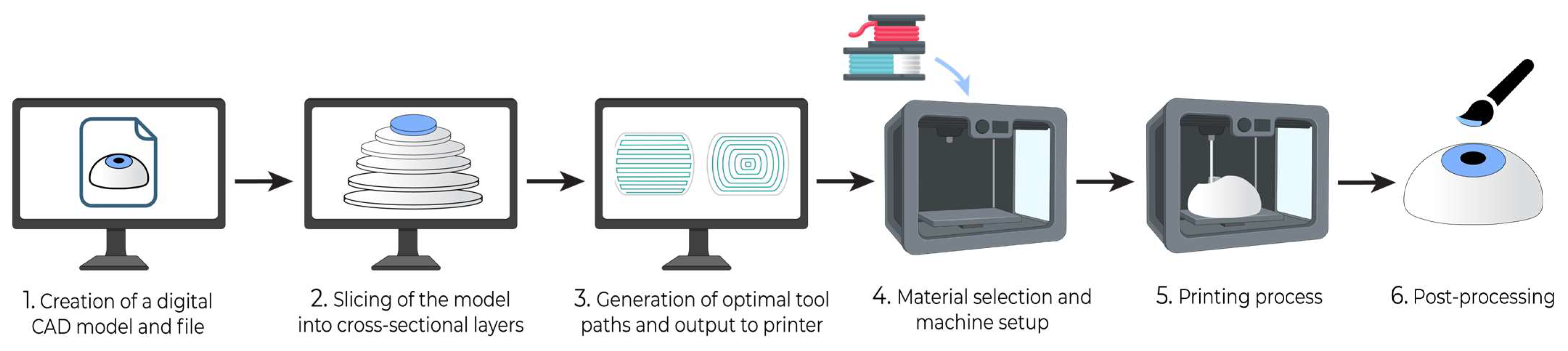
Figure 1.
General build steps for 3D printing.
3. Applications of 3D Printing in Ophthalmology
3.1. Three-Dimensional Printing in Ophthalmic Implants and Prosthetics
Occasionally, patients who have suffered a serious orbito–ocular injury, including fractures, eye cancers, or trauma, necessitate the surgical extraction of the affected eye(s) [25][11]. In that regard, three surgical interventions may be performed, depending on the indication. First, evisceration is a procedure that entails the removal of the viscera of the eye while maintaining the extraocular muscles, Tenon’s capsule, scleral coat, and optic nerve intact [26][12]. It is most indicated in cases of serious intraocular infection, causing pain and blindness [26][12]. Enucleation, on the other hand, implicates the surgical extraction of the complete eyeball by cleaving the optic nerve and the extraocular muscles, and it is performed to treat malignant ocular neoplasms, as well as irreversible traumas [26][12]. Finally, exenteration is considered the most invasive of the three surgeries, involving the eradication of all orbital material, including bone tissue, for the definitive treatment of advanced malignancies [26][12]. Following one of these operations, an orbital implant will be fitted and placed into the eyeless cavity to restore structure and adequate volume, followed by a visual prosthesis above the graft to achieve the look of a normal eye. In the traditional method, the surgeon establishes the approximate size of the orbital implant prior to surgery visually, based on the estimations of the patient’s fracture site anatomy, combined with computed tomography-measured orbital volume [27][13]. However, the imprecisions of this technique can increase the risk of the implant not appropriately fitting the anatomy of the fracture, which can, in turn, cause ophthalmic complications, such as enophthalmos, diplopia, and displacement of the implant [28][14]. With the emergence of three-dimensional printing technology to create templates, customization of the implants in terms of size, shape, and contour can be more accurately achieved. In fact, 3D printing permits the creation of customized implants precisely fitting the fracture site or eye socket to better treat blowout fractures and congenital abnormalities, as well as aid evisceration and enucleation procedures. A 2018 study by Kang et al. described the review of 11 patients who underwent orbital wall reconstruction for orbital floor and medial wall fractures with the assistance of custom 3D-printed orbital implant templates. The templates were used per-operatively to shape implants before their insertion into the fracture site [28][14]. Quantitative analysis of patient outcomes was based on the CT measurements of the volume of orbital tissue confined in the bony orbit. This analysis revealed that all 11 patients presented no postoperative complications, and a statistically significant reduction was noted between the pre-operative and postoperative orbital tissue volumes of the affected orbit (24.00 ± 1.74 vs. 22.31 ± 1.90 cm3; p = 0.003) [28][14]. In contrast, the contralateral unaffected orbit and the reconstructed affected orbit had similar volumes (22.01 ± 1.60 vs. 22.31 ± 1.90 cm3; p = 0.182), demonstrating successful fracture repair [28][14]. Kormann et al. (2019) evaluated the biocompatibility of 3D-printed spherical orbital implants made of photocurable resin in 10 patients who underwent evisceration of painful blind eyes. To do so, they measured systemic toxicity by comparing serum biochemical markers before surgery and at 12 months after. Local toxicity was evaluated by assessing the signs of socket inflammation one month postoperatively, as well as changes in implant size on CT scans at two and 12 months after surgery [29][15]. None of the ten patients presented signs of infection, inflammation, exposure, or extrusion of the implant, and no changes in implant size were revealed on computed tomography imaging [29][15]. Thus, this phase-1 clinical study attested to the biocompatibility of photocurable resin for SLA 3D-printed human orbital implants.
Ocular prostheses are custom-made eye models that can be used for cosmetic rehabilitation in individuals who were either born with a congenital eye abnormality or who have had their eyes removed as part of the treatment of various ocular diseases [30,31][16][17]. Many factors must be considered to create a realistic and symmetric prosthesis for the anophthalmic patient, which include the position, size, contour, and color of the device, as well as its weight, comfort, cosmesis, and motility [31,32][17][18]. Traditional methods of creating a customized prosthesis, which have remained relatively unchanged over the past century, are labor-intensive, time-consuming, and expensive, generally requiring the skills of an experienced ocularist or craftsman to hand-paint the iris and sclera [30][16]. In comparison, three-dimensional printing technology allows the fabrication of a high-quality, customized design based on the patient’s eye anatomy in a significantly shorter time than for a conventional hand-made prosthesis [30,31][16][17]. In 2016, Ruiters et al. successfully 3D printed and fitted a patient-specific prosthesis for a 68-year-old male with anophthalmia secondary to evisceration surgery [33][19]. In this case, a digital 3D model of the patient’s anophthalmic cavity was obtained using a CT scan, which is different from the traditional method of creating a mold by injecting impression material into the patient’s eye socket. The 3D-printed prosthesis was dimensionally accurate but lacked color, so postprocessing had to be performed to add iris and scleral characteristics [33][19]. Likewise, in Alam et al.’s 2017 study, a white artificial eye was created using computer-aided design (CAD) and rapid 3D printing based on a CT scan of a wax model of two patients’ orbits [32][18]. It was compared with a conventional custom-made prosthesis (CMP), and the CAD prosthesis was found to necessitate much lesser manufacturing time (2.5 h versus 10 h for the CMP). It weighed less (2.9 g compared to 4.4 g for the CMP), and it was subjectively more comfortable for both patients [32][18]. Kim et al. (2021) have also demonstrated a sublimation transfer printing technique that can reproduce the appearance of the contralateral healthy eye on a printed prosthetic without the need for manual painting [34][20].
Another form of non-invasive ocular prosthetics that can be three-dimensionally printed consists of eyelid crutches for the treatment of ptosis. Blepharoptosis can be quite debilitating for patients, especially in terms of vision deterioration and eye dryness caused by the difficulty in completely closing the affected eye. Despite surgical options to correct myopathy-induced eyelid drooping, there is a potential risk of recurrence after surgery [35][21]. Sun et al. (2018) reported using 3D printing to design and fabricate custom-fit crutches as an alternative and inexpensive therapeutic option for recurring ptosis. Not only were the printed eyelid crutches more affordable to fabricate than standard ones, but they could be easily removed and adjusted as well. Furthermore, after five months of usage, patients’ vision was reported to have improved, and proper eye closure was possible [35][21]. Therefore, large-scale 3D printing and adoption of these devices can be contemplated to increase accessibility for patients struggling with ptosis in the near future.
Finally, macular buckles are surgically implanted devices that are used to treat an uncommon complication of myopia called myopic foveoschisis, which can increase the risk of retinal detachment and subsequent visual impairment [36,37][22][23]. Despite these devices being available in different shapes and sizes, their fit is frequently not perfect because of their generic structure. To remedy these issues, Pappas et al. (2020) created a custom macular buckle by 3D printing biocompatible polymers, specifically polyether ether ketone (PEEK), based on the exact 3D geometry of a patient’s eye from CT imaging [38][24]. This customized device has the potential to decrease the complications associated with surgical intervention by minimizing the manipulation of extraocular muscles, sclera, and blood vessels by the surgeon. However, it was determined that the mechanical durability of the 3D-printed parts of the buckle was suboptimal in comparison to the pieces that were injection molded. Thus, it will be important to optimize the device’s strength in upcoming studies, and alternatives to radiation imaging, such as laser scanning microscopy or B-scan ocular ultrasound, will have to be explored to increase the safety of this technique [38][24].
In summary, there is evidently increasing promise for the widespread production of orbital implants and ocular prostheses using three-dimensional printing technology. Nevertheless, some limitations and challenges need to be addressed. First, even with the use of 3D printing, there are manual tasks that are still necessary to create customized ocular prostheses for patients, which can be time-consuming and make it only a semi-automated process [31][17]. These may include the impression mold of the patient’s anophthalmic cavity, as well as the painting and polishing of the prosthesis [31][17]. In most studies, an ocularist was also needed to paint the external eye anatomy. However, there has been an instance where a photograph of the pupil, iris, and conjunctival blood vessels of the contralateral normal eye of the patient was taken using a slit lamp and subsequently printed on transfer paper, which was then transferred to the 3D printed prosthesis by sublimation [31][17]. This entire process added approximately an hour in total to the 3D manufacturing of the prosthesis, in contrast to the several hours required by a skilled ocularist to reproduce the iris and blood vessels manually. Another limitation is the cost of the printing itself. Direct 3D printing of titanium implants for orbital reconstruction can cost thousands of USD per implant [39][25]. However, the creation of orbital templates to shape standard implants, along with the continued maturation of 3D printing technologies, can significantly reduce costs while maintaining satisfactory results for patients [28][14]. Moreover, additional studies and clinical trials with larger sample sizes, as well as longer follow-up intervals, are required to corroborate the efficacy, cost reduction, cosmetic outcome, and patient comfort associated with 3D-printed ocular prostheses and orbital implants [28,31][14][17].
3.2. Educational and Anatomical Models
Conventional images of ocular anatomy may fail to contain sufficient detail and dimensional information for optimal teaching of medical students and residents. Three-dimensional printed anatomical models can recreate the detailed structure of the eye and its intricacies to enhance ophthalmology education. The orbit is one of the most complicated anatomical regions in the human body, with a high degree of individual variation. Despite this, 3D printed models of the orbit visualizing both bony structure and soft tissue can provide ocular anatomy details that improve learning, compared to textbook and computer-based learning methods [40][26]. In a meta-analysis of studies evaluating 3D printed models for anatomy education, Ye et al. found that 3D printed models resulted in faster answering time (−0.61, p < 0.05) and greater response accuracy among students in comparison to conventional methods such as cadaver and 2D learning [5].
A different randomized study by Wu et al. found educational value in a 3D-printed ocular model for teaching ophthalmoscopy to medical students [41][27]. The researchers randomly assigned 92 medical students to either a model-assisted training group, where students practiced on the simulated eye and with peers, or a traditional training group, where students only practiced with peers. After equal training time, both groups were assessed on their ability to see the fundus and determine the cup-to-disk ratio in patients. In the model-assisted training group, 43/46 (93.48%) students correctly determined the ratio, while in the control group, only 21/46 (45.65%) students saw the fundus and determined the correct ratio. The significant difference in skill acquisition suggests value in 3D printed ocular models for facilitating student learning before progressing to real patients. However, the study was non-blind due to the nature of the intervention, which may have mildly influenced student performance [41][27]. Although 3D printed anatomical models have benefited undergraduate medical learners, the literature has shown inconclusive benefits for resident physician learners and no statistical difference compared to training with 3D visual imaging [42][28].
However, 3D printed models remain a promising alternative to cadaveric specimens due to greater availability and reduced cost, especially for learners in geographically isolated areas [40][26]. They also offer greater anatomical reproducibility and can flexibly simulate diverse pathologies processed from CT or MRI images. The non-perishable nature of 3D printed models makes them further suited for cost-effectively teaching specific and complex conditions [7,43][7][29]. A systematic review of 15 randomized trials evaluating 3D models for anatomy education found that models enhance knowledge acquisition, and most students are interested in utilizing 3D systems for learning compared to traditional methods [44][30]. In addition to education, 3D printing can be used for advanced visualization and diagnosis of ophthalmic conditions. Maloca et al. utilized optical coherence tomography (OCT) to image the architecture of choroidal vessels and tumors. These imaging data were then used to create 3D choroid models with FDM and SLA printing that illustrated the interactions of tumors with the vascular network of the choroid [45][31]. As choroidal tumors can disrupt the delicate vascular supply of the retina, 3D models that visualize and localize tumors can aid clinicians in diagnosis and treatment planning. Limitations regarding 3D printed anatomical models include the pliability and texture of many printable materials not being representative of real specimens. It is particularly challenging to replicate the anisotropic and viscoelastic variability of anatomy consisting of multiple tissues [23][32]. Finally, to produce accurate 3D anatomical models, models must be based on high-quality scans of specimens or prosections to ensure educational value [40][26].
3.3. Surgical Planning and Training
Surgical planning in ophthalmology is assisted by the ability of 3D-printed models to visualize patient anatomy and simulate operative procedures. Three-dimensional printed surgical simulators can provide visualized and tactile surgical training for procedures such as orbital surgery and keratoplasty. Notably, training with fracture models has been shown to enhance performance during operations and improve patient-reported functional outcomes [46][33]. A study by Famery et al. examined the performance of surgeons on Descemet membrane endothelial keratoplasty (DMEK) using a 3D printed platform. Human donor corneas were mounted on artificial anterior chambers with a 3D-printed iris, which allowed the adjustment of pupil size and anterior chamber depth for accurate simulation and modifiable surgical difficulty [8]. The realistic model enabled surgeons to practice all unfolding techniques that would be used in real surgery, and all surgeons, including beginners, completed the simulation with well-oriented grafts verified by OCT. Thus, the model demonstrates the value of 3D printing for surgical training with the ability to accurately simulate ophthalmic surgery.
Furthermore, 3D-printed surgical guides can be leveraged to benefit operative procedures directly. Fan et al. compared the clinical success of 3D-assisted orbital reconstructions compared to traditional surgery with a study of 56 patients [47][34]. The 3D-assisted technique utilized orbital models true to patients’ anatomy, which allowed surgeons to conduct pre-operative planning. Surgeons also used the printed template to shape polyethylene-titanium mesh to better fit the patient during surgery. Compared to the control group, which underwent reconstruction without 3D printing, the 3D group achieved significantly shorter operating time (75.34 ± 15.68 min vs. 95.37 ± 22.19 min; p < 0.05). Furthermore, the postoperative clinical results were superior in the 3D group, with significantly lower enophthalmos and a lower percentage of superior sulcus deformity [47][34]. Similar studies have printed custom 3D models of patients’ orbits mapped from CT imaging and used these models to create pre-shaped implants for orbital fracture reconstruction [48,49][35][36]. Specifically, a titanium mesh was shaped and cut to size for patients according to their orbital anatomy, as represented in PolyJet-printed and resin 3D models. The use of the model reduced operation time, improved enophthalmos, and contributed to successful treatment outcomes while being relatively inexpensive to implement [48,49][35][36]. In addition to helping shape implants, 3D printing can directly create implantable polycaprolactone (PCL) mesh for use in the treatment of orbital wall fractures. A retrospective review of patient cases has shown that 3D-printed biodegradable PCL mesh enables ideal repair of orbital wall fractures with reliable stabilization and a low complication rate [50][37]. Furthermore, 3D-printed models of eyes with intraocular tumors have demonstrated utility in guiding radiosurgery by enabling physicians to visualize tumor location physically and design more accurate stereotactic radiation therapy [13][38].
Challenges identified for 3D printing in surgical planning include the length of time required to design and print models (10–14 h) and the increased logistical complexity of treatment [48][35]. Overall, the long time required to image patient anatomy and print physical models makes 3D surgical preplanning less feasible for highly time-sensitive surgeries and emergent procedures. However, these are not common in ophthalmology, which enables the field to uniquely harness the benefits of 3D-printed surgical guides and planning models. A key advantage of 3D printers for surgical planning is the ability to visualize precise patient anatomy and diverse surgical cases using a single device. Applying 3D printing to aid surgeries can also enhance traditional procedures and provide templates to shape implantable devices [47][34]. The studies reviewed show that surgical preplanning models improve procedural readiness among surgeons and improve patient outcomes [48][35]. Three-dimensional printed simulators also present a unique opportunity for assessing and maintaining surgical competence for students and physicians in a controlled environment [8]. Finally, by demonstrating pathological conditions and surgical procedures on realistic models, patients can better understand their own conditions, which involves them in their care and helps build patient-provider trust. Although 3D rapid prototyping in ophthalmic surgery is promising, continued innovation in materials that accurately resemble ocular tissues is needed to allow for more realistic surgical simulation going forward.
3.4. Drug-Delivery Systems and 4D Printing
Three-dimensional printing technology has evolved considerably and rapidly in the last decade, and novel derivatives of 3D printing are currently in the works. For instance, four-dimensional printing, which encompasses time, is surfacing as a manufacturing method for medical materials and devices [51][39]. This novel technology empowers biomaterials to change their physical and functional properties over time, which promises to advance tissue engineering and enable new drug-delivery platforms [52][40]. For example, 4D-printed biomaterials can modify their structure in response to changes in temperature, pH, and ion concentrations, even after printing. These biomaterials can further undergo modifications to their function as the cells they contain mature [51][39]. Because of these characteristics, designs produced using 4D printing are designated as smart materials [53][41]. In healthcare, materials able to adapt their properties, functionality, and shape as a function of time have expanded to implants, targeted drug delivery, and complex surgery.
3.4.1. Drug-Eluting Implants
One potential 4D printing application in ophthalmology involves the use of 4D-printed hydrogel-based microneedles as a drug-delivery system that reacts to environmental stimuli [52][40]. Microneedles can be used as a simple, minimally invasive drug-delivery procedure with very little pain sensation [54,55,56][42][43][44]. These devices were classically used for the transdermal administration of different pharmaceutical agents, but with the impressive recent developments in the field of microtechnology, some studies have shown great promise for their use in the treatment of ophthalmic diseases [56,57][44][45]. The shape of the microneedles used in the 4D printing process can change if they are dissolved, under pressure, or cured with UV rays, which benefits the utility of precision drug-eluting implants [52][40]. Notably, implants that include intraocular pressure-responsive biomaterials can release IOP-lowering drugs at controlled times to treat glaucoma [51][39]. These 4D drug-eluting implants could be an alternative to topical eye drops, which include various hypotensive antiglaucoma agents [58][46]. These locally acting medications, which act by decreasing the production of aqueous humor or by increasing its drainage through the trabecular meshwork and the uveoscleral outflow, have their limiting factors [58][46]. They show low patient compliance, attributable to difficulties in their administration and ocular irritation or discomfort, particularly in the elderly [59][47]. Other limitations include their brief therapeutic time and less than 5% bioavailability, which is explained by various precorneal factors, such as blinking, solution drainage, and tear film clearance [60][48]. Furthermore, the anatomical barriers of the cornea, conjunctiva, and sclera reduce drug absorption, necessitating frequent high-concentration doses of eye drops [61,62][49][50]. Therefore, with a 4D-printed implant releasing an antiglaucoma agent inside the eye, these limiting factors could be compensated.
Another promising therapeutic application of drug-delivery systems is for the treatment of retinal vascular diseases. Currently, the most widespread clinical therapy for diabetic retinopathy and its potential complication, diabetic macular edema (DME), as well as wet age-related macular degeneration (AMD) and macular edema secondary to retinal vein occlusion (RVO), consists of repeated intravitreal injections of an anti-vascular endothelial growth factor, or anti-VEGF, medication [63,64,65][51][52][53]. Anti-VEGF agents, such as bevacizumab, inhibit a crucial growth factor in the pathogenesis of neovascularization, the process in which new immature blood vessels are formed [59,63][47][51]. This pathophysiological characteristic is seen in proliferative diabetic retinopathy, where hyperglycemia promotes retinal neovascularization by regulating the synthesis of VEGF and PEDF (pigment epithelium-derived factor). In wet AMD, angiogenesis instead occurs in the choroid layer behind the retina of the eye [59,63][47][51]. These capillaries are very fragile and can easily leak exudate, which can precipitate vitreous or subretinal hemorrhage, fibrosis, and tractional retinal detachment. In the worst-case scenario, capillary bleeding can cause irreversible retina damage, vision impairment, and even blindness [59,64][47][52]. Consequently, minimizing this potentially harmful neovascular process is of greatest importance in these retinal and macular diseases, and anti-VEGF injections have proven to be the gold standard in preserving and improving visual acuity for disease of the retina [63,65][51][53]. However, to deliver adequate quantities of an anti-VEGF medication to the posterior segment of ocular tissues and to counterbalance the rapid clearing of the medication from the vitreous body, frequent intravitreal injections are necessary, especially in the first few months of therapy [59][47]. These recurring treatments, in combination with frequent visits to an ophthalmologist’s office every four to eight weeks, can constitute a significant physical, emotional, and economic burden not only on patients but also on their caretakers, as well as on healthcare professionals [63,66,67,68][51][54][55][56]. Moreover, it was demonstrated that these repeated injections can increase the risk of retinal detachment, hemorrhage, and intraocular inflammation [59,63,69][47][51][57]. To avoid these potential complications, Won et al. (2020) developed a drug-loaded rod, also called a drug rod, using a flexible coaxial 3D printing technique, which was implanted in rat vitreous using a minimally invasive small-gauge needle and delivered bevacizumab and dexamethasone in a time-controlled manner into the vitreal cavity [63][51]. The drug rod incorporated an external shell that was 3D printed using polycaprolactone and bevacizumab (PCL-BEV), and the interior core contained an infusion of alginate and dexamethasone (ALG-DEX). Coaxial printing was achieved with a multiple-head 3D bioprinter and a set of coaxial nozzles containing numerous combinations of core/shell needles [63][51]. Specifically, the PCL-BEV ink, formed by the dilution of both substances in dichloromethane (DCM), was distributed in the shell needle of a coaxial nozzle, while a hydrogel was simultaneously released by the core needle of the same nozzle. The interior core ALG-DEX bioink was assembled by diffusing sodium alginate in deionized water and combining this solution with dexamethasone. During the printing process, the PCL-BEV shell rapidly solidified due to evaporation of the DCM solvent, and the hydrogel core was removed by deionized water and replaced by the administration of the ALG-DEX ink to form the drug rod. Subsequent in vitro and in vivo studies proved that the structural design and the biomaterials comprising the rod allowed the controlled release of both bevacizumab and dexamethasone, as well as extended their therapeutic duration, compared to the conventional intravitreal treatments. In fact, the drug rod was able to continuously deliver BEV for 60 days, in contrast to the injected BEV’s 2-week half-life. Additionally, choroidal neovascularization was inhibited by the drug rod over a 4-week evaluation period in a rat model, whereas the intravitreal bevacizumab was able to suppress angiogenesis for only 2 weeks [63][51]. Therefore, not only is this technology able to reduce the side effects associated with intravitreal injections, but it can improve compliance by increasing the drugs’ release period, as well as making their administration more bearable for patients since the rod’s implantation process is a much less invasive technique [63,70][51][58]. Nonetheless, more studies will be required to evaluate their safety for use in humans, as well as determine the most efficacious combinations of drugs, doses, routes, and drug-release patterns that will better stabilize degenerative retinal diseases while maintaining a minimal side-effect profile.
3.4.2. Drug-Eluting Contact Lenses
3D printed drug-eluting contact lenses are another novel technique that has the potential to revolutionize the treatment of various ocular conditions, including keratoconjunctivitis sicca, or dry-eye disease, age-related macular degeneration, and glaucoma [71][59]. In fact, these lenses are not only useful to correct visual acuity deficits and refractory errors but can also deliver medications in a controlled manner and offer greater bioavailability to the eye’s surface compared to standard eye drops [59,72][47][60]. When a contact lens is deposited onto the cornea, the tear film is divided into two components: the pre-lens tear film (PLTF), in which drugs are absorbed by the conjunctiva or gain access to the systemic circulation by entering the canaliculi, and the post-lens tear film (POLTF), where medications diffuse through the cornea using a direct approach [59][47]. Drug-eluting contact lenses can be manufactured using 3D printing techniques such as FDM, as demonstrated by Mohamdeen et al. [73][61]. They fabricated lenses from a blend of ethylene-vinyl acetate copolymer (EVA) and polylactic acid (PLA) using hot melt extrusion. Integrated with the lens filament was timolol maleate (TML), a glaucoma medication that reduces intraocular fluid production. An EVA/PLA/TML ratio of 84:15:1 (wt./wt.) was found to be ideal for printability, lens integrity, and drug release. The 3D printed lens released loaded TML over 3 days but only eluted 35% of the total drug. The authors reason that sustained release was not achieved due to slow diffusion from the polymer matrix, and further work is needed to optimize drug release [73][61]. Methods for optimizing ocular drug delivery include integrating different nanocarriers into the lenses’ composition, such as polymer nanoparticles, liposomes, micelles, and microemulsions [74][62]. These nanomaterials are also important not only to prevent the enzymatic degradation of the drug but also to minimize the possible medication leak during its storing and sterilization processes [71,75][59][63]. Factors that will require more consideration in the future to create safe and effective drug-delivery systems using contact lenses include biocompatibility, oxygen permeability, tensile strength, optical transparency, sterilization, and storage, without forgetting patient comfort [76,77][64][65]. Future development of smart and drug-eluting lenses leveraging 3D printing could offer a minimally invasive and safe route for ocular drug delivery [78][66].
3.5. Four-Dimensional Orbital Implants
An additional 4D printing prospect concerns the treatment of enophthalmic invagination. Enophthalmos is described as the posterior displacement of the normal-sized ocular globe within the orbit following an anteroposterior plane [79,80][67][68]. This relative shift can occur following orbital trauma or not, and it is corrected by filling the orbital volume with an implant, which in turn can reinstate facial symmetry [80][68]. Unfortunately, the current implant devices that are used lack precision and capability to fill the increased volume, and they necessitate large surgical incisions to be correctly implanted [79][67]. Shape memory polymers (SMPs) are printable stimuli-responsive smart materials and can present in different temporary and permanent shapes when exposed to heat, electrical fields, light, magnetic fields, and solutions [79,81,82,83][67][69][70][71]. Shape memory polyurethane specifically has an adjustable transition temperature that is determined by the melting temperature of its soft segment, and its firmer segment dictates its permanent structure [84,85][72][73]. It also possesses satisfactory mechanical characteristics, antithrombotic properties, and biocompatibility that make it a safe material for the production of personalized ophthalmic implants in the near future [79][67]. Deng et al. (2022) created an orbital stent based on CT reconstruction technology, and 4D printed a shape memory polyurethane composite to treat enophthalmos [79][67]. In its compressed temporary state, the stent was implanted in a minimally invasive fashion into rabbits before thermal stimulation enabled the assumption of its permanent shape. The volume filling ability was nearly 150% greater compared to two commercially available implants, which included Medpor, made of porous polyethylene, and absorbable plates [79][67]. Thus, printed stents leveraging shape-changing materials can enable precise treatment of enophthalmos.
3.6. Adaptive Optics
Adaptive optics refers to a non-invasive technique that corrects optical aberrations using deformable mirrors, which can be applied to the eye and accurately depict the retina’s cells [86,87][74][75]. This concept was first proposed in 1953 by American astrophysicist Horace Babcock to refine the telescopic images of distant stars, which lacked precision and clarity because of the optical deviations caused by Earth’s atmospheric turbulence [86,87][74][75]. Likewise, with the eye’s anatomy being very complex and made of different tissues, the differences in the refractive indexes of these ocular tissues create wavefront chromatic and monochromatic aberrations when light rays exit the eye [87][75]. Monochromatic aberrations are further classified as being low-order or high-order. Lower-order aberrations include refractive errors, such as myopia and hypermetropia, as well as astigmatism, and despite them being of greater importance and much more prevalent, they are easily corrected with spherical and cylindrical lenses, respectively [87,88][75][76]. On the other hand, higher-order aberrations, like keratoconus, are far less common but are more arduous to correct [88][76]. In 1997, Liang et al. were able to put together a fundus camera, combined with a Shack-Hartmann wavefront sensor (SHWS) and a deformable mirror, to produce high-quality images of the retina at its cellular level, specifically the cone photoreceptors, by overcoming the higher-order monochromatic aberrations [89][77]. This was the first application of adaptive optics in ophthalmology, and it paved the way for numerous studies assessing the different retinal components in vivo, such as photoreceptors, retinal pigment epithelial cells, and microvascular anomalies [86][74]. An in-depth examination of retinal cells and anomalies can provide a better understanding of the diseases affecting the retina, as well as help in their diagnosis before substantial damage occurs [87][75]. Thus, existing treatments for retinal pathologies could be administered as a preventive measure, and new therapeutic modalities could be developed to better control or even stop the progression of these diseases. Adaptive optics can also be combined with other retinal imaging techniques, such as flood illumination ophthalmoscopy (FIO), scanning laser ophthalmoscopy (SLO), optical coherence tomography (OCT), fundus fluorescein angiography (FFA), and indocyanine green angiography (ICG), and complement their findings [86][74]. There are four main components to the standard adaptive optics equipment:
- (1)
-
A wavefront sensor to qualify and quantify the optical aberrations in the light reflected by the eye;
- (2)
-
A deformable mirror to correct the identified abnormalities;
- (3)
-
A control system to calculate the necessary correction amount and to provide feedback, and;
- (4)
-
A processing device to create an image based on the corrected waveform.In terms of 3D and 4D printing, López-Valdeolivas et al. (2017) described the 4D manufacture of a liquid crystalline elastomer (LCE)-embedded polydimethylsiloxane (PDMS) actuator that could be used for adaptive optics, owing to this material’s flexibility, effortless handling, translucency, low weight, absence of toxicity and cost-effectiveness [90][78]. Hammer et al. (2019) created a biomimetic phantom that corresponded to the human retina to evaluate the performance of adaptive optics. The retinal model mimicked the photoreceptor mosaic, respecting the arrangement and the size of cells, and its cone photoreceptors were 3D fabricated using the two-photon polymerization technique [91][79]. This model eye was designed to allow imaging with SLO and OCT with the potential to help in the evaluation of AO device functioning. In the future, the retinal images generated from AO could be 3D-printed to be used as educational models for surgical planning purposes, and eventually, 3D bioprinting of retinal cells could also be achieved for possible transplantation. Nonetheless, there are challenges associated with the use of 3D-printed adaptive optics in a clinical setting. This includes its very high purchase price and the potential difficulty in obtaining satisfactory quality images, especially in eyes that present diverse abnormalities, such as dryness, cataracts, corneal scars, vitreous debris, or involuntary ocular movements like nystagmus [86][74]. Other limitations concern the very narrow zone that can be imaged at a time, meaning that some areas of retinal pathology could be omitted, and the very time-consuming and complex analysis of the images [86][74].In short, there are many novel technologies currently being studied in ophthalmology, which include different 3D and 4D printed drug-delivery systems, such as implants, shape memory polymers, and adaptive optics imaging. All these innovations have the potential to aid the treatment of ocular diseases, but these are not without limitations and side effects. Therefore, additional studies will be necessary in the near future to attest to their safety for human use.
References
- Kholgh Eshkalak, S.; Rezvani Ghomi, E.; Dai, Y.; Choudhury, D.; Ramakrishna, S. The role of three-dimensional printing in healthcare and medicine. Mater. Des. 2020, 194, 108940.
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616.
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290.
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473.
- Ye, Z.; Dun, A.; Jiang, H.; Nie, C.; Zhao, S.; Wang, T.; Zhai, J. The role of 3D printed models in the teaching of human anatomy: A systematic review and meta-analysis. BMC Med. Educ. 2020, 20, 335.
- Lichtenberger, J.P.; Tatum, P.S.; Gada, S.; Wyn, M.; Ho, V.B.; Liacouras, P. Using 3D Printing (Additive Manufacturing) to Produce Low-Cost Simulation Models for Medical Training. Mil. Med. 2018, 183 (Suppl. S1), 73–77.
- Jones, D.B.; Sung, R.; Weinberg, C.; Korelitz, T.; Andrews, R. Three-Dimensional Modeling May Improve Surgical Education and Clinical Practice. Surg. Innov. 2016, 23, 189–195.
- Famery, N.; Abdelmassih, Y.; El-Khoury, S.; Guindolet, D.; Cochereau, I.; Gabison, E.E. Artificial chamber and 3D printed iris: A new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2019, 97, e179–e183.
- Wang, Y.; Wang, J.; Ji, Z.; Yan, W.; Zhao, H.; Huang, W.; Liu, H. Application of Bioprinting in Ophthalmology. Int. J. Bioprint. 2022, 8, 552.
- Ruiz-Alonso, S.; Villate-Beitia, I.; Gallego, I.; Lafuente-Merchan, M.; Puras, G.; Saenz-del-Burgo, L.; Pedraz, J.L. Current Insights into 3D Bioprinting: An Advanced Approach for Eye Tissue Regeneration. Pharmaceutics 2021, 13, 308.
- Chalasani, R.; Poole-Warren, L.; Conway, R.M.; Ben-Nissan, B. Porous Orbital Implants in Enucleation: A Systematic Review. Surv. Ophthalmol. 2007, 52, 145–155.
- Chen, X.Y.; Yang, X.; Fan, X.L. The Evolution of Orbital Implants and Current Breakthroughs in Material Design, Selection, Characterization, and Clinical Use. Front. Bioeng. Biotechnol. 2022, 9, 800998.
- Kwon, M.S.; Shin, H.J. Comparison of Orbital Reconstructive Effect between Customized Orbital Implants Using Three-Dimensional Printed Templates and Conventional Manual-Bending Implants in Blowout Fracture Surgery. Appl. Sci. 2023, 13, 9012.
- Kang, S.; Kwon, J.; Ahn, C.J.; Esmaeli, B.; Kim, G.B.; Kim, N.; Sa, H.-S. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018, 32, 1864–1870.
- Kormann, R.B.; Mörschbächer, R.; Moreira, H.; Akaishi, P. A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration. Arq. Bras. Oftalmol. 2019, 82, 471–475.
- Raizada, K.; Rani, D. Ocular prosthesis. Contact Lens Anterior Eye J. Br. Contact Lens Assoc. 2007, 30, 152–162.
- Ko, J.; Kim, S.H.; Baek, S.W.; Chae, M.K.; Yoon, J.S. Semi-automated fabrication of customized ocular prosthesis with three–dimensional printing and sublimation transfer printing technology. Sci. Rep. 2019, 9, 2968.
- Alam, M.S.; Sugavaneswaran, M.; Arumaikkannu, G.; Mukherjee, B. An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: A pilot study. Orbit 2017, 36, 223–227.
- Ruiters, S.; Sun, Y.; de Jong, S.; Politis, C.; Mombaerts, I. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br. J. Ophthalmol. 2016, 100, 879–881.
- Kim, B.R.; Kim, S.H.; Ko, J.; Baek, S.W.; Park, Y.K.; Kim, Y.J.; Yoon, J.S. A Pilot Clinical Study of Ocular Prosthesis Fabricated by Three-dimensional Printing and Sublimation Technique. Korean J. Ophthalmol. 2021, 35, 37–43.
- Sun, M.G.; Rojdamrongratana, D.; Rosenblatt, M.I.; Aakalu, V.K.; Yu, C.Q. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit 2019, 38, 342–346.
- Baba, T.; Ohno-Matsui, K.; Futagami, S.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Tokoro, T.; Mochizuki, M. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am. J. Ophthalmol. 2003, 135, 338–342.
- Gaucher, D.; Haouchine, B.; Tadayoni, R.; Massin, P.; Erginay, A.; Benhamou, N.; Gaudric, A. Long-term Follow-up of High Myopic Foveoschisis: Natural Course and Surgical Outcome. Am. J. Ophthalmol. 2007, 143, 455–462.e1.
- Pappas, G.; Vidakis, N.; Petousis, M.; Maniadi, A. Individualized Ophthalmic Exoplants by Means of Reverse Engineering and 3D Printing Technologies for Treating High Myopia Complications with Macular Buckles. Biomimetics 2020, 5, 54.
- Vehmeijer, M.; van Eijnatten, M.; Liberton, N.; Wolff, J. A Novel Method of Orbital Floor Reconstruction Using Virtual Planning, 3-Dimensional Printing, and Autologous Bone. J. Oral Maxillofac. Surg. 2016, 74, 1608–1612.
- Adams, J.W.; Paxton, L.; Dawes, K.; Burlak, K.; Quayle, M.; McMenamin, P.G. 3D printed reproductions of orbital dissections: A novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br. J. Ophthalmol. 2015, 99, 1162–1167.
- Wu, C.; Luo, M.; Liu, Y.; Dai, R.; Zhang, M.; Zhong, Y.; Chen, Y. Application of a 3D-printed eye model for teaching direct ophthalmoscopy to undergraduates. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2361–2368.
- Ye, Z.; Jiang, H.; Bai, S.; Wang, T.; Yang, D.; Hou, H.; Zhang, Y.; Yi, S. Meta-analyzing the efficacy of 3D printed models in anatomy education. Front. Bioeng. Biotechnol. 2023, 11, 1117555.
- Michaels, R.; Witsberger, C.A.; Powell, A.R.; Koka, K.; Cohen, K.; Nourmohammadi, Z.; Green, G.E.; Zopf, D.A. 3D printing in surgical simulation: Emphasized importance in the COVID-19 pandemic era. J. 3D Print. Med. 2021, 5, 5–9.
- Ardila, C.M.; González-Arroyave, D.; Zuluaga-Gómez, M. Efficacy of three-dimensional models for medical education: A systematic scoping review of randomized clinical trials. Heliyon 2023, 9, e13395.
- Maloca, P.M.; Tufail, A.; Hasler, P.W.; Rothenbuehler, S.; Egan, C.; Ramos de Carvalho, J.E.; Spaide, R.F. 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol. 2019, 97, e313–e316.
- Jaksa, L.; Pahr, D.; Kronreif, G.; Lorenz, A. Development of a Multi-Material 3D Printer for Functional Anatomic Models. Int. J. Bioprint. 2021, 7, 420.
- Masada, K.M.; Cristino, D.M.; Dear, K.A.; Hast, M.W.; Mehta, S. 3-D Printed Fracture Models Improve Resident Performance and Clinical Outcomes in Operative Fracture Management. J. Surg. Educ. 2023, 80, 1020–1027.
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057.
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234.
- Zhang, X.; Chen, W.; Luo, T.-Y.; Ma, J.; Dong, Z.; Cao, G.; Xu, J.-K.; Liu, B.-Y.; Zhang, Q.-R.; Zhang, S.-L. Application of Three-Dimensional Printing Technology in the Orbital Blowout Fracture Reconstruction. J. Craniofac. Surg. 2019, 30, 1825–1828.
- Kim, S.Y. Application of the three-dimensionally printed biodegradable polycaprolactone (PCL) mesh in repair of orbital wall fractures. J. Cranio-Maxillofacial Surg. 2019, 47, 1065–1071.
- Furdová, A.; Sramka, M.; Thurzo, A.; Furdová, A. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin. Ophthalmol. 2017, 11, 267–271.
- Tan, G.; Ioannou, N.; Mathew, E.; Tagalakis, A.D.; Lamprou, D.A.; Yu-Wai-Man, C. 3D printing in Ophthalmology: From medical implants to personalised medicine. Int. J. Pharm. 2022, 625, 122094.
- Willemen, N.G.A.; Morsink, M.A.J.; Veerman, D.; da Silva, C.F.; Cardoso, J.C.; Souto, E.B.; Severino, P. From oral formulations to drug-eluting implants: Using 3D and 4D printing to develop drug delivery systems and personalized medicine. Bio-Des. Manuf. 2022, 5, 85–106.
- Shahbazi, M.; Jäger, H.; Ettelaie, R.; Mohammadi, A.; Asghartabar Kashi, P. Multimaterial 3D printing of self-assembling smart thermo-responsive polymers into 4D printed objects: A review. Addit. Manuf. 2023, 71, 103598.
- Singh, T.R.R.; Mcmillan, H.; Mooney, K.; Alkilani, A.Z.; Donnelly, R.F. 6—Microneedles for Drug Delivery and Monitoring. In Microfluidic Devices for Biomedical Applications; Li, X., Zhou, Y., Eds.; Woodhead Publishing: Delhi, India, 2013; pp. 185–230.
- Han, D.; Morde, R.S.; Mariani, S.; La Mattina, A.A.; Vignali, E.; Yang, C.; Barillaro, G.; Lee, H. 4D Printing of a Bioinspired Microneedle Array with Backward-Facing Barbs for Enhanced Tissue Adhesion. Adv. Funct. Mater. 2020, 30, 1909197.
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307.
- Gadziński, P.; Froelich, A.; Wojtyłko, M.; Białek, A.; Krysztofiak, J.; Osmałek, T. Microneedle-based ocular drug delivery systems—Recent advances and challenges. Beilstein J. Nanotechnol. 2022, 13, 1167–1184.
- Tătaru, C.P.; Purcărea, V.L. Antiglaucoma pharmacotherapy. J. Med. Life 2012, 5, 247–251.
- Sapowadia, A.; Ghanbariamin, D.; Zhou, L.; Zhou, Q.; Schmidt, T.; Tamayol, A.; Chen, Y. Biomaterial Drug Delivery Systems for Prominent Ocular Diseases. Pharmaceutics 2023, 15, 1959.
- Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T.J.; Banerjee, S.; Banerjee, S.K. Protein PEGylation for cancer therapy: Bench to bedside. J. Cell Commun. Signal. 2019, 13, 319–330.
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360.
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133.
- Won, J.Y.; Kim, J.; Gao, G.; Kim, J.; Jang, J.; Park, Y.-H.; Cho, D.-W. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases. Acta Biomater. 2020, 116, 174–185.
- Nikkhah, H.; Karimi, S.; Ahmadieh, H.; Azarmina, M.; Abrishami, M.; Ahoor, H.; Alizadeh, Y.; Behboudi, H.; Daftarian, N.; Dehghan, M.H.; et al. Intravitreal Injection of Anti-vascular Endothelial Growth Factor Agents for Ocular Vascular Diseases: Clinical Practice Guideline. J. Ophthalmic Vis. Res. 2018, 13, 158–169.
- Reibaldi, M.; Fallico, M.; Avitabile, T.; Marolo, P.; Parisi, G.; Cennamo, G.; Furino, C.; Lucenteforte, E.; Virgili, G. Frequency of Intravitreal Anti-VEGF Injections and Risk of Death: A Systematic Review with Meta-analysis. Ophthalmol. Retina 2022, 6, 369–376.
- Reitan, G.; Kjellevold Haugen, I.B.; Andersen, K.; Bragadottir, R.; Bindesbøll, C. Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway. Clin. Ophthalmol. 2023, 17, 1465–1474.
- Jørstad, Ø.K.; Steffensen, L.A.; Eriksen, K.; Bragadóttir, R.; Moe, M.C. Thirteen years of intravitreal anti-vascular endothelial growth factor therapy: The promises and burdens of a paradigm shift told from the perspective of the largest retina service in Norway. Acta Ophthalmol. 2020, 98, 774–779.
- Kristiansen, I.S.; Haugli Bråten, R.; Jørstad, Ø.K.; Moe, M.C.; Sæther, E.M. Intravitreal therapy for retinal diseases in Norway 2011–2015. Acta Ophthalmol. 2020, 98, 279–285.
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Drug delivery to the retina: Challenges and opportunities. Expert. Opin. Biol. Ther. 2003, 3, 45–56.
- Thrimawithana, T.R.; Young, S.; Bunt, C.R.; Green, C.; Alany, R.G. Drug delivery to the posterior segment of the eye. Drug Discov. Today 2011, 16, 270–277.
- Mutlu, Z.; Shams Es-haghi, S.; Cakmak, M. Recent Trends in Advanced Contact Lenses. Adv. Healthc. Mater. 2019, 8, 1801390.
- Zhang, X.; Cao, X.; Qi, P. Therapeutic contact lenses for ophthalmic drug delivery: Major challenges. J. Biomater. Sci. Polym. Ed. 2020, 31, 549–560.
- Mohamdeen, Y.M.G.; Tabriz, A.G.; Tighsazzadeh, M.; Nandi, U.; Khalaj, R.; Andreadis, I.; Boateng, J.S.; Douroumis, D. Development of 3D printed drug-eluting contact lenses. J. Pharm. Pharmacol. 2022, 74, 1467–1476.
- Bal-Öztürk, A.; Özcan-Bülbül, E.; Gültekin, H.E.; Cecen, B.; Demir, E.; Zarepour, A.; Cetinel, S.; Zarrabi, A. Application of Convergent Science and Technology toward Ocular Disease Treatment. Pharmaceuticals 2023, 16, 445.
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118.
- Lanier, O.L.; Christopher, K.G.; Macoon, R.M.; Yu, Y.; Sekar, P.; Chauhan, A. Commercialization challenges for drug eluting contact lenses. Expert. Opin. Drug Deliv. 2020, 17, 1133–1149.
- Kim, T.Y.; Lee, G.-H.; Mun, J.; Cheong, S.; Choi, I.; Kim, H.; Hahn, S.K. Smart contact lens systems for ocular drug delivery and therapy. Adv. Drug Deliv. Rev. 2023, 196, 114817.
- Rykowska, I.; Nowak, I.; Nowak, R. Soft Contact Lenses as Drug Delivery Systems: A Review. Molecules 2021, 26, 5577.
- Deng, Y.; Yang, B.; Zhang, F.; Liu, Y.; Sun, J.; Zhang, S.; Zhao, Y.; Yuan, H.; Leng, J. 4D printed orbital stent for the treatment of enophthalmic invagination. Biomaterials 2022, 291, 121886.
- Saravanan, A.; Patel, B.C. Enophthalmos. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK563300/ (accessed on 13 November 2023).
- Liu, Y.; Zhang, W.; Zhang, F.; Lan, X.; Leng, J.; Liu, S.; Jia, X.; Cotton, C.; Sun, B.; Gu, B.; et al. Shape memory behavior and recovery force of 4D printed laminated Miura-origami structures subjected to compressive loading. Compos. Part B Eng. 2018, 153, 233–242.
- Zhao, W.; Zhang, F.; Leng, J.; Liu, Y. Personalized 4D printing of bioinspired tracheal scaffold concept based on magnetic stimulated shape memory composites. Compos. Sci. Technol. 2019, 184, 107866.
- Wang, W.; Du, L.; Xie, Y.; Zhang, F.; Li, P.; Xie, F.; Wan, X.; Pei, Q.; Leng, J.; Wang, N. Bioinspired four-dimensional polymeric aerogel with programmable temporal-spatial multiscale structure and functionality. Compos. Sci. Technol. 2021, 206, 108677.
- Yoo, D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater. Sci. Eng. C 2013, 33, 1759–1772.
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta BBA—Gen. Subj. 2018, 1862, 936–945.
- Akyol, E.; Hagag, A.M.; Sivaprasad, S.; Lotery, A.J. Adaptive optics: Principles and applications in ophthalmology. Eye 2021, 35, 244–264.
- Mohankumar, A.; Tripathy, K. Adaptive Optics. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK589704/ (accessed on 13 November 2023).
- Gill, J.S.; Moosajee, M.; Dubis, A.M. Cellular imaging of inherited retinal diseases using adaptive optics. Eye 2019, 33, 1683–1698.
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A 1997, 14, 2884–2892.
- López-Valdeolivas, M.; Liu, D.; Broer, D.J.; Sánchez-Somolinos, C. 4D Printed Actuators with Soft-Robotic Functions. Macromol. Rapid Commun. 2018, 39, 1700710.
- Hammer, D.X.; Kedia, N.; Liu, Z.; Tam, J.; Agrawal, A. Phantom-Based Model Eyes for Adaptive Optics Performance Assessment. In Applied Industrial Optics 2019; OSA Technical Digest; Optica Publishing Group: Washington, DC, USA, 2019; p. W2A.2.
More
