Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Wojciech Rozek and Version 2 by Peter Tang.
The tick-borne encephalitis virus (TBEV) is the arboviral etiological agent of tick-borne encephalitis (TBE), considered to be one of the most important tick-borne viral diseases in Europe and Asia.
- tick-borne encephalitis
- TBEV
- arbovirus
- Flaviviridae
- Ixodes
- vector-borne
1. Introduction
Tick-borne encephalitis (TBE) is an arboviral disease caused by the TBE virus (TBEV), a member of the Flaviviridae family, as are the etiological agents of dengue fever, yellow fever, and Japanese encephalitis. Tick-borne encephalitis is a serious health problem in Europe and Asia [1][2][1,2]. In recent years, a rise in the incidence of TBE as well as an expansion of the geographical range of the disease have been evident. In 2020, 24 European Union/European Economic Area (EU/EEA) member countries reported 3817 cases of TBE [3]. The virus is transmitted between ticks, animals, and humans, so it can be considered in the context of a one health perspective [4]. Humans are incidental and dead-end hosts infected mainly through the bites of hard ticks. Another route of TBEV transmission may be the ingestion of unpasteurized milk or dairy products from infected animals [5].
2. Virus Structure
The tick-borne encephalitis virus is spherical or quasi-spherical, lipid-enveloped, and approximately 50 nm in diameter, and it contains a positive, single-stranded RNA genome that acts as mRNA for translation. Although the approximate size and shape of TBEV and other flaviviruses are estimated, there are variations in size due to factors such as the genetic diversity in the virus population, changes during the maturation process, and the methods used for the imaging and analysis of virions [6]. The virion is composed of three structural proteins: the envelope (E), membrane (M), and capsid (C) proteins (Figure 1). Seven non-structural (NS) proteins—NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5—have also been identified in infected cells. Non-structural proteins play an important role in virus replication, the processing of the structural proteins, and the modulation of host cell function. The M glycoprotein is primarily synthesized as a precursor (prM) that interacts with glycoprotein E and protects its fusion loop from premature activation [7][8][7,8]. The nucleocapsid consists of the genome and the C protein and is surrounded by the viral envelope, which consists of both M and E glycoproteins and host-cell-derived lipids. Glycoprotein E is the major antigen of TBEV and is responsible for receptor binding and membrane fusion [8][9][8,9]. The glycoprotein-E-coding gene is commonly sequenced and analyzed, but a pairwise distance analysis indicated that it has evolutionary patterns distinct from other TBEV genomic regions [10].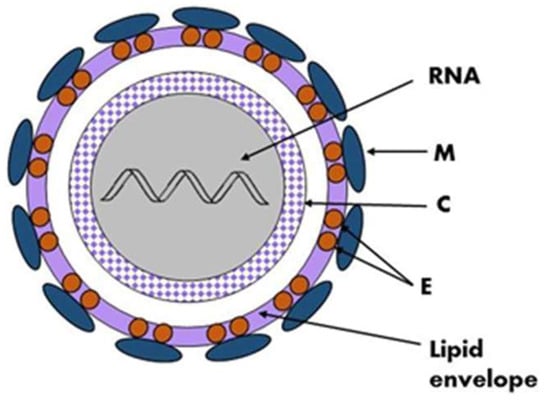
Figure 1. Schematic structure of the TBEV virion, the viral genome (RNA) and the C protein form a nucleocapsid surrounded by a lipid envelope in which glycoproteins E and M are embedded.
3. Phylogenetic Analysis of Circulating Virus Subtypes
Traditionally, TBEVs have been classified into three subtypes: the European (TBEV-Eu), the Siberian (TBEV-Sib), and the Far-Eastern (TBEV-FE). Recently, Baikalian (TBEV-Bkl) and Himalayan (TBEV-Him) subtypes have been distinguished [15][16][17][15,16,17]. The assumption that if the open reading frame nucleotide sequence of two viruses differs by less than 10%, the two viruses belong to the same subtype guided the recent analyses, and they pointed to seven subtypes of TBEV: TBEV-Eu, TBEV-Sib, TBEV-FE, TBEV-Ob (TBEV-2871), TBEV-Him, TBEV-Bkl-1 (178-79), and TBEV-Bkl-2 (886-84) (Figure 2) [10]. Another phylogenetic analysis using the Nextstrain framework and based on more than 220 complete TBEV genomes proposed TBEV-Bkl1, TBEV-Bkl-2, and TBEV-Him as separate clades in addition to the three major subtypes TBEV-Eu, TBEV-Sib, and TBEV-FE [18]. The viral strains within the three main subtypes of TBEV, TBEV-Eu, TBEV-Sib, and TBEV-FE are believed to be descended from a common ancestor and have evolved independently. Recent studies on TBEV strains isolated near Lake Baikal in Russia, TBEV-Bkl-2 (886-84), have shown that these strains have a mosaic genome: some parts are more closely related to viruses from the Siberian group, while others are more closely related to the Far-Eastern group. Therefore, the Baikalian subtype of TBEV is postulated as evident of recombination between the Siberian and Far-Eastern subtypes [19]. The phylogenetic groups of TBEV may differ in their clinical presentation. For the European subtype, the fatality rate has been estimated at below 2% [20]. The disease caused by European subtypes of TBEV is usually biphasic with a viremic phase associated with a fever and myalgia, and in some patients, is followed by neurological symptoms of varying severity [1]. The Far-Eastern subtype is considered the most pathogenic, with a mortality rate estimated at up to 40% by some authors [21][22][21,22]. The Siberian subtype typically results in a less severe disease than that caused by the TBEV-FE subtype, with a fatality rate of 6–8%, and it may be associated with chronic TBE [23]. The TBEV-Ob (2871) strain was isolated from Ixodes pavlovskyi, and it has not been detected in humans, so its pathogenicity to humans is unknown. The virulence of the strain has been confirmed in laboratory mice. According to the classification based on the invasiveness index, the TBEV-Ob strain belongs to the group of the most common strains from Western Siberia [24]. Him-TBEV was detected in a wild rodent, Marmota himalayana, at the Qinghai–Tibet Plateau in China. An analysis of 17 amino acid residues associated with the pathogenicity of TBEV showed that Him-TBEV shares nine substitutions that are specific to pathogenic strains and five substitutions that are specific to strains isolated from subclinical cases. The pathogenic-associated amino acid substitution profile of the Him-TBEV strain is similar to the low-pathogenic TBEV Oshima strain [16]. The ability of TBEV-Bkl-2 (886-84) to cause lethal focal forms of encephalitis, as well as the results of laboratory tests, indicate the high pathogenic potential of this group [25]. However, assigning a certain level of pathogenicity to strains of a given subtype may be misleading. Some studies have shown that different strains within certain TBEV subtypes may show a variable virulence [26][27][26,27].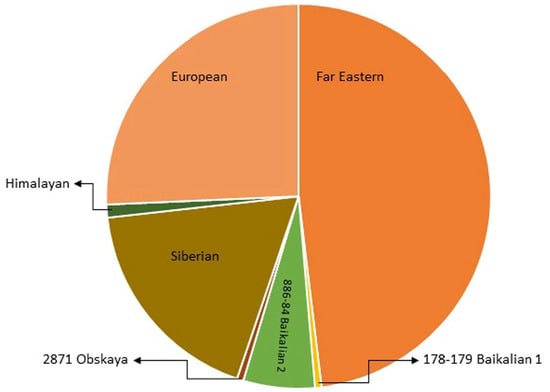
Figure 2. The pie chart shows the abundance of identified TBEV strains within subtypes.  Far Eastern group: 88 TBEV strains;
Far Eastern group: 88 TBEV strains;  European: 47 strains;
European: 47 strains;  Siberian: 33 strains;
Siberian: 33 strains;  886-84 Baikalian 2: 11 strains;
886-84 Baikalian 2: 11 strains;  Himalayan: 2 strains;
Himalayan: 2 strains;  178-179 Baikalian 1: 1 strain and
178-179 Baikalian 1: 1 strain and  Obskaya 2971: 1 strain [10][16][19][24][10,16,19,24].
Obskaya 2971: 1 strain [10][16][19][24][10,16,19,24].
 Far Eastern group: 88 TBEV strains;
Far Eastern group: 88 TBEV strains;  European: 47 strains;
European: 47 strains;  Siberian: 33 strains;
Siberian: 33 strains;  886-84 Baikalian 2: 11 strains;
886-84 Baikalian 2: 11 strains;  Himalayan: 2 strains;
Himalayan: 2 strains;  178-179 Baikalian 1: 1 strain and
178-179 Baikalian 1: 1 strain and  Obskaya 2971: 1 strain [10][16][19][24][10,16,19,24].
Obskaya 2971: 1 strain [10][16][19][24][10,16,19,24].4. TBEV Reservoirs, Vectors, and Transmission
Hard ticks of the family Ixodidae act both as vectors and reservoirs of TBEV. Ixodes ricinus occurs especially in central, northern, and eastern Europe, and I. persulcatus is found in parts of the Baltic States, Finland, Russia, and Siberia [28]. Field studies and experimental findings indicate that other species of ticks might also be effective TBEV vectors. Natural infections with TBEV were reported in 16 species of ixodid ticks more than 30 years ago [6]. Currently, at least eight species are known to be able to transmit the TBE virus, and so far, the virus has been isolated from at least 14 other species [29]. In Central Europe, TBEV has been revealed in some species of “hard” ticks: Ixodes persulcatus, Ixodes ricinus, Ixodes hexagonus [30], Ixodes arboricola [31], Haemaphysalis punctate [32], Haemaphysalis concinna [33], Dermacentor marginatus, and Dermacentor reticulatus [34]. Ixodes gibbosus is considered a marginal vector in the Mediterranean region [32]. Nosek et al. experimentally proved the vector competence of Haemaphysalis inermis for TBEV [35]. TBEV circulates in small, geographically defined areas, so-called “natural foci”. This cycle involves ticks as vectors and small rodents, insectivores, birds, and large mammals as hosts (Figure 3). Ticks can become infected by feeding on viremic hosts (viremic transmission) or by co-feeding with an infected tick (non-viremic transmission) [36]. Other ways of transmitting the virus include vertical transovarial transmission (via eggs laid by an infected female) and transstadial transmission (between developmental stages of ticks). Horizontal sexual transmission may take place between both ticks and warm-blooded hosts [37]. The transstadial and transovarial transmission of TBEV co-exist and take place simultaneously along with sexual, non-viremic, and other transmission modes.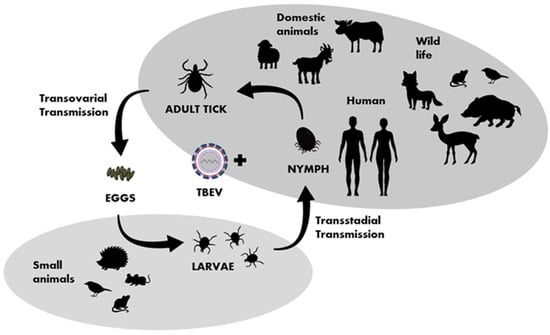
Figure 3.
Transmission of TBEV in the life cycle of ixodid ticks, shaded fields indicate the host group characteristic for particular developmental stages of ticks.
