Chronic progressive external ophthalmoplegia (CPEO) is the most common manifestation of mitochondrial diseases and is characterized by bilateral symmetrical progressive ptosis and reduced ocular motility. CPEO can be isolated or accompanied by a clinical feature of systemic involvement of mitochondrial dysfunction (CPEO plus syndrome). Mitochondrial disorders generally affect tissues with high metabolic demand, such as the central and peripheral nervous systems, heart, adrenal glands, renal tubules, skeletal muscles, and the eye. In CPEO, the ocular findings of ptosis and ophthalmoplegia occur due to the inability of the abnormal mitochondria to supply an adequate amount of ATP due to defective oxidative phosphorylation. The extraocular muscles are particularly susceptible due to their high mitochondrial volume and lower mutational threshold. Their susceptibility is expressed in multiple mitochondrial disorders, highlighting the significance of examining other manifestations in patients with PEO.
- CPEO
- DNA deletion
- Kearns–Sayre syndrome
- Pearson syndrome
- Leigh syndrome
- MELAS
1. Introduction
2. CPEO
In its isolated form, CPEO is typically a sporadic disorder characterized by progressive bilateral ptosis and ophthalmoparesis [9] (Figure 1). Ptosis examination yields poor levator palpebrae superioris (LPS) muscle function, where eyelid excursion is often less than 8–10 mm rather than the normal ≥12 mm. Slowed, incomplete, and omnidirectional saccades can be a subtle early clinical sign that is frequently missed. Later on, as the disease progresses, ophthalmoplegia becomes more evident. The often-symmetric nature of the disease means that patients do not have diplopia, and reports of manifest strabismus with diplopia in CPEO patients are rare [14,15][10][11]. Retinal examination could reveal pigmentary retinopathy that is typical in Kearns–Sayre syndrome, characterized as salt and pepper retinopathy, where clumps of retinal pigment epithelium (RPE) alternate with areas devoid of RPE [16][12]. However, these retinal changes rarely harbor field defects or a change in visual acuity.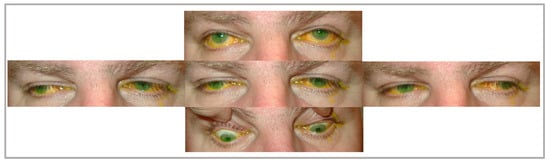
3. Kearns–Sayre Syndrome
Kearns–Sayre syndrome is a syndrome of CPEO and pigmentary retinopathy, with onset before the age of 20 as well as one of the following features: a complete heart block, cerebellar ataxia, dementia, deafness, short stature, endocrine abnormalities, and cerebrospinal fluid (CSF) protein of more than 100 mg/dL. If the diagnostic criteria are not met, the patient is termed “CPEO plus” or “KSS-minus” [20][16]. When a patient presents with CPEO before the age of 20, they should be evaluated with mtDNA sequencing followed by regular ophthalmologic assessments and screening for systemic signs and symptoms. A muscle biopsy can be performed to look for the ragged red fibers. The fundoscopic examination reveals pigmentary retinopathy that should be distinguished from retinitis pigmentosa since they might share similar symptoms like mildly reduced night vision and visual acuity. Retinitis pigmentosa typically affects the peripheral or the mid-peripheral retina with a bone spicule pattern, whereas KSS affects the posterior retina with a salt and pepper pattern [21][17]. It is essential to perform an electrocardiogram on these patients to rule out a complete heart block. Endocrine abnormalities affecting the adrenals, parathyroid, and hypothalamus can present with diabetes mellitus, growth hormone deficiency, and short stature [22,23][18][19]. Orbicularis oculi muscle weakness can impair eyelid closure, and frontalis weakness can affect eyelid elevation. Dysphagia is a rare presentation of KSS and may result from upper esophageal sphincter dysfunction and reduced peristalsis in the pharynx and upper esophagus, as observed in a manometric study of a case report by Shaker et al. [24][20]. No definitive treatment option is available for KSS. Symptomatic treatment includes correction of CPEO, treating heart blocks with pacemakers with a long-term cardiology follow-up, correction of endocrine abnormalities, and cochlear implants in cases of hearing loss.4. Pearson Syndrome
Pearson syndrome (PS), also known as Pearson marrow–pancreas syndrome, is a rare fatal multisystemic mitochondrial disease due to deletions in mtDNA, and it typically affects infants. Ophthalmologic manifestations include corneal endothelial dysfunction, ptosis, CPEO, and mild peripheral pigmentary retinopathy [25][21]. It is also characterized by refractory sideroblastic anemia, lactic acidosis, and exocrine pancreatic dysfunction. It can also present with vacuolization of hematopoietic precursors, pancytopenia, failure to thrive, diarrhea, hypospadias, cleft lip palate, diabetes mellitus, renal tubular dysfunction, hepatic failure, enteropathy, and rashes [26][22]. Cardiac manifestations, such as bundle branch blocks and supraventricular tachycardia, have been reported; however, cardiac involvement is not yet a part of the major criterion of the disease [27][23]. Usually, premature death at three years of age occurs due to infection from neutropenia or metabolic crisis. Thus, early diagnosis is essential in improving the poor prognosis for these patients. The diagnosis of Pearson syndrome is challenging due to the atypical presentation in infancy. It can be confirmed via mtDNA sequencing and observing multiple deletions of varying lengths [28][24]. Interestingly, these single large-scale mtDNA deletions can also be found in young patients with CPEO and KSS. They, therefore, form a continuous spectrum of diseases termed “mtDNA deletion syndromes”, supported by reports of a KSS-like phenotype in PS survivors [29][25]. Treatment for Pearson syndrome is supportive and may include blood transfusions, iron chelating therapy, pancreatic replacement therapy, and prompt detection and management of cardiac dysfunction. Bone marrow transplant has been tested and, unfortunately, yielded poor outcomes [26,30][22][26].5. Leigh Syndrome
Leigh syndrome is a fatal, progressive neurodegenerative disease that typically manifests in infants and young children of 3 months to 2 years of age [31][27]. It can be caused by multiple mtDNA deletions as well as nDNA defects in more than 75 different monogenic causes, most commonly by the SURF1 variant [32,33][28][29]. The clinical features of LS vary, with the most common characteristics, according to a meta-analysis by Chang et al., being developmental delay, hypotonia, respiratory dysfunction, epilepsy, reduced feeding, and weakness [34][30]. The ocular features of LS include nystagmus, ptosis, ophthalmoplegia, strabismus, pigmentary retinopathy, and optic atrophy [34,35][30][31]. Common cardiac abnormalities are hypertrophic or dilated cardiomyopathy and conduction defects such as Wolff–Parkinson–White syndrome [36,37][32][33]. Consensus on the clinical diagnosis is yet to be determined; however, LS is suspected through the hallmarks of the disease along with findings suggestive of brainstem dysfunction in addition to T2 weighted brain MRI lesions and accessory laboratory findings [34][30]. Brain MRI findings typically show bilateral symmetrical supra-tentorial (basal ganglia, thalamus, and sub-thalamus) and/or infra-tentorial (brainstem and dentate nuclei) lesions. A study by Ardissone et al. presented a predominating basal ganglia involvement of 90.2%. They also showed that both supra and infra-tentorial involvement is dominant in cases of both mtDNA (74%) and -nDNA (67%) variants, while isolated infra-tentorial variants are rare [38][34]. Extensive research is being conducted to find genetic correlations with MRI findings of LS. For example, a retrospective cohort found significant associations between the SURF1 variant and inferior olivary nuclei lesions [39][35]. Abnormal laboratory findings may yield elevated blood, urine, and CSF lactate levels. Additional deficiencies may be observed in respiratory chain complexes through enzyme assays and pyruvate dehydrogenase complex [40][36]. However, these laboratory findings are not consistently present. Therefore, confirmatory tests with genetic assays are required for a definitive diagnosis and the identification of specific variants of LS [41][37].6. MELAS
MELAS, or mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (SLEs) are associated with A to G RNA transfer mutation (Leu (UUR)) in the most commonly m.3243A>G mutation [42,43][38][39]. The clinical presentations vary widely, usually in childhood, with neurological symptoms that include SLEs, sensorineural hearing loss, and cognitive impairment associated with diffuse white matter injury. Less commonly, it can present with gastrointestinal manifestations that include gastric perforation, ischemic colitis, segmental ileal paralysis, pseudo-obstruction, or megacolon. Endocrine manifestations, such as diabetes mellitus, have also been reported in MELAS [44][40]. Ophthalmologic manifestations of MELAS include hemianopia and cortical blindness from SLEs, nystagmus, cataracts, CPEO, optic atrophy, salt and pepper pigmentary retinopathy, and macular degeneration [45][41]. The transient SLEs of the disease are characterized by nausea, vomiting, a migraine-like headache, encephalopathy, and focal seizures with or without neurological deficits. The exact pathogenic mechanism for these episodes is yet to be determined; however, three theories have been postulated. The first is insufficient energy due to mitochondrial dysfunction, supported by the increase in lactate peaks and decreased N-acetyl aspartate peaks of the occipital regions in brain magnetic resonance spectroscopy (MRS) [46][42]. The second is nitric oxide (NO) deficiency, which usually regulates oxygenation and blood flow. This hypothesis is supported by a reduction in NO metabolites during acute attacks and an increase in NO synthase inhibitors in the COX-negative fibers of MELAS patients [47][43]. The third theory is mitochondrial angiopathy, an accumulation of mitochondria in the smooth muscle cells and endothelial cells of small cerebral arteries leading to the narrowing of the lumen of blood vessels and reducing perfusion [48][44]. MRI findings of SLE exhibit stroke-like lesions (SLLs) that are usually differentiated from other pathologies by initially observing cortical and deep white matter lesions, in addition to occipital and parietal lobe lesions or lesions not confined to arterial territories. PWI/ASL can also show hyperperfused lesions, and MRS exhibits lactate peaks [49][45]. Another distinctive finding in neuroimaging was reported in some cases of MELAS as cerebellar lesions SLLs [49,50][45][46]. Since MELAS is associated with reduced levels of citrulline and arginine, which are NO precursors, and decreased NO that contributes to SLEs, supplement replacement with arginine was proposed. A systematic review by Argudo et al. concluded that the studies conducted showed promising results in managing SLEs [51][47]. Acute phase management consists of giving an intravenous dose of 500 mg/kg/day or 10 g/m2 in 24 h for 3–5 days. Whereas chronically, 150–300 mg/kg/day (maximum of 500 mg) is used instead [52][48]. A study conducted by Pek et al. using induced pluripotent stem cell-derived endothelial cells vouched for edaravone, a potent antioxidant, to be used for improving the vascular function in MELAS since it scavenges ROS and inhibits the inflammatory response in cerebrovascular diseases, which L-arginine and citrulline do not tackle [42][38]. For treating epilepsy, levetiracetam is considered to be the first-line anticonvulsant in mitochondrial encephalomyopathy due to the mitochondrial toxicity of other anticonvulsant agents [53][49].References
- Yu-Wai-Man, P.; Clements Al Nesbitt, V.; Griffiths, P.G.; Gorman, G.S.; Schaefer, A.M.; Turnbull, D.M.; Taylor, R.W.; McFarland, R. A national epidemiological study of chronic progressive external ophthalmoplegia in the United Kingdom—Molecular genetic features and neurological burden. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5109.
- Kearns, T.P.; Sayre, G.P. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: Unusual syndrome with histologic study in one of two cases. AMA Arch. Ophthalmol. 1958, 60, 280–289.
- Olson, W.; Engel, W.K.; Walsh, G.O.; Einaugler, R. Oculocraniosomatic neuromuscular disease with “ragged-red” fibers. Arch. Neurol. 1972, 26, 193–211.
- Zintz, R.; Villiger, W. Electron microscopic findings in 3 cases of chronic progressive ocular muscular dystrophy. Ophthalmologica 1967, 153, 439–459.
- Reske-Nielsen, E.; Lou, H.C. Lowes, MProgressive external ophthalmoplegia. Evidence for a generalised mitochondrial disease with a defect in pyruvate metabolism. Acta Ophthalmol. 1976, 54, 553–573.
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331, 717–719.
- Zeviani, M.; Servidei, S.; Gellera, C.; Bertini, E.; DiMauro, S.; DiDonato, S. An autosomal dominant disorder with multiple deletions of mitochondrial DNA starting at the D-loop region. Nature 1989, 339, 309–311.
- Kaukonen, J.; Juselius, J.K.; Tiranti, V.; Kyttälä, A.; Zeviani, M.; Comi, G.P.; Keränen, S.; Peltonen, L.; Suomalainen, A. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 2000, 289, 782–785.
- Thangaraj, K.; Khan, N.A.; Govindaraj, P.; Meena, A.K. Mitochondrial disorders: Challenges in diagnosis & treatment. Indian J. Med. Res. 2015, 141, 13–26.
- Chatzistefanou, K.I.; Brouzas, D.; Asproudis, I.; Tsina, E.; Droutsas, K.D.; Koutsandrea, C. Strabismus surgery for diplopia in chronic progressive external ophthalmoplegia. Int. Ophthalmol. 2019, 39, 213–217.
- Richardson, C.; Smith, T.; Schaefer, A.; Turnbull, D.; Griffiths, P. Ocular motility findings in chronic progressive external ophthalmoplegia. Eye 2005, 19, 258–263.
- Pfeffer, G.; Sirrs, S.; Wade, N.K.; Mezei, M.M. Multisystem Disorder in Late-Onset Chronic Progressive External Ophthalmoplegia. Can. J. Neurol. Sci. 2011, 38, 119–123.
- Sun, M.G.; Rojdamrongratana, D.; Rosenblatt, M.I.; Aakalu, V.K.; Yu, C.Q. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit 2019, 38, 342–346.
- Ahn, J.; Kim, N.J.; Choung, H.K.; Hwang, S.W.; Sung, M.; Lee, M.J.; Khwarg, S.I. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br. J. Ophthalmol. 2008, 92, 1685–1688.
- Rajabi, M.T.; Tabatabaie, S.Z.; Rajabi, M.B.; Abrishami, Y.; Hosseini, S.S.; Oestreicher, J. Management of myogenic ptosis in chronic progressive external ophtalmoplegia. Iran. J. Neurol. 2014, 13, 185–187.
- Shemesh, A.; Margolin, E. Kearns Sayre Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Ortiz, A.; Arias, J.; Cárdenas, P.; Villamil, J.; Peralta, M.; Escaf, L.C.; Ortiz, J. Macular findings in Spectral Domain Optical Coherence Tomography and OCT Angiography in a patient with Kearns–Sayre syndrome. Int. J. Retin. Vitr. 2017, 3, 24.
- Kang, Y.X.; Wang, Y.J.; Zhang, Q.; Pang, X.H.; Gu, W. A case of hypopituitarism accompanying Kearns–Sayre syndrome treated with human chorionic gonadotropin: A case report and literature review. Andrologia 2017, 49, e12711.
- Ng, Y.S.; Lim, A.Z.; Panagiotou, G.; Turnbull, D.M.; Walker, M. Endocrine Manifestations and New Developments in Mitochondrial Disease. Endocr. Rev. 2021, 43, 583–609.
- Katsanos, K.H.; Nastos, D.; Noussias, V.; Christodoulou, D.; Kappas, A.; Tsianos, E.V. Manometric study in Kearns–Sayre syndrome. Dis. Esophagus 2001, 14, 63–66.
- Vadhul, R.; Halbach, C.S.; Areaux, R.G., Jr.; Berry, S.; Hou, J.H. Endothelial dysfunction in a child with Pearson marrow-pancreas syndrome managed with Descemet stripping automated endothelial keratoplasty using a suture pull-through technique. Digit. J. Ophthalmol. 2019, 25, 59–64.
- Shoeleh, C.; Donato, U.M.; Galligan, A.; Vitko, J. A Case Report on Pearson Syndrome with Emphasis on Genetic Screening in Patients Presenting with Sideroblastic Anemia and Lactic Acidosis. Cureus 2023, 15, e33963.
- Jennifer, M.S.; Cortez, D. Pearson marrow-pancreas syndrome with cardiac conduction abnormality necessitating prophylactic pacemaker implantation. Ann. Noninvasive Electrocardiol. 2020, 25, e12681.
- Reddy, J.; Jose, J.; Prakash, A.; Devi, S. Pearson syndrome: A rare inborn error of metabolism with bone marrow morphology providing a clue to diagnosis. Sudan. J. Paediatr. 2019, 19, 161–164.
- Broomfield, A.; Sweeney, M.G.; Woodward, C.E.; Fratter, C.; Morris, A.M.; Leonard, J.V.; Abulhoul, L.; Grunewald, S.; Clayton, P.T.; Hanna, M.G.; et al. Paediatric single mitochondrial DNA deletion disorders: An overlapping spectrum of disease. J. Inherit. Metab. Dis. 2015, 38, 445–457.
- Faraci, M.; Cuzzubbo, D.; Micalizzi, C.; Lanino, E.; Morreale, G.; Dallorso, S.; Castagnola, E.; Schiaffino, M.C.; Bruno, C.; Rossi, A.; et al. Allogeneic bone marrow transplantation for Pearson’s syndrome. Bone Marrow Transplant. 2007, 39, 563–565.
- Saini, A.G.; Chatterjee, D.; Bhagwat, C.; Vyas, S.; Attri, S.V. Leigh syndrome in an infant: Autopsy and histopathology findings. Autops. Case Rep. 2021, 11, e2021334.
- Lake, N.J.; Compton, A.G.; Rahman, S.; Thorburn, D.R. Leigh syndrome: One disorder, more than 75 monogenic causes. Ann. Neurol. 2016, 79, 190–203.
- Kistol, D.; Tsygankova, P.; Krylova, T.; Bychkov, I.; Itkis, Y.; Nikolaeva, E.; Mikhailova, S.; Sumina, M.; Pechatnikova, N.; Kurbatov, S.; et al. Leigh Syndrome: Spectrum of Molecular Defects and Clinical Features in Russia. Int. J. Mol. Sci. 2023, 24, 1597.
- Chang, X.; Wu, Y.; Zhou, J.; Meng, H.; Zhang, W.; Guo, J. A meta-analysis and systematic review of Leigh syndrome: Clinical manifestations, respiratory chain enzyme complex deficiency, and gene mutations. Medicine 2020, 99, e18634.
- Han, J.; Lee, Y.-M.; Kim, S.M.; Han, S.Y.; Lee, J.B. Ophthalmological manifestations in patients with Leigh syndrome. Br. J. Ophthalmol. 2015, 99, 528–535.
- Sofou, K.; de Coo, I.F.M.; Ostergaard, E.; Isohanni, P.; Naess, K.; De Meirleir, L.; Tzoulis, C.; Uusimaa, J.; Lönnqvist, T.; Bindoff, L.A.; et al. Phenotype-genotype correlations in Leigh syndrome: New insights from a multicentre study of 96 patients. J. Med. Genet. 2018, 55, 21–27.
- Brecht, M.; Richardson, M.; Taranath, A.; Grist, S.; Thorburn, D.; Bratkovic, D. Leigh Syndrome Caused by the MT-ND5 m.13513G>A Mutation: A Case Presenting with WPW-Like Conduction Defect, Cardiomyopathy, Hypertension and Hyponatraemia. JIMD Rep. 2015, 19, 95–100.
- Ardissone, A.; Bruno, C.; Diodato, D.; Donati, A.; Ghezzi, D.; Lamantea, E.; Lamperti, C.; Mancuso, M.; Martinelli, D.; Primiano, G.; et al. Clinical, imaging, biochemical and molecular features in Leigh syndrome: A study from the Italian network of mitochondrial diseases. Orphanet J. Rare Dis. 2021, 16, 413.
- Alves, C.A.P.F.; Teixeira, S.R.; Martin-Saavedra, J.S.; Gonçalves, F.G.; Russo, F.L.; Muraresku, C.; McCormick, E.M.; Falk, M.J.; Zolkipli-Cunningham, Z.; Ganetzky, R.; et al. Pediatric Leigh Syndrome: Neuroimaging Features and Genetic Correlations. Ann. Neurol. 2020, 88, 218–232.
- Baertling, F.; Rodenburg, R.J.; Schaper, J.; Smeitink, J.A.; Koopman, W.J.H.; Mayatepek, E.; Morava, E.; Distelmaier, F. A guide to diagnosis and treatment of Leigh syndrome. J. Neurol. Neurosurg. Psychiatry 2014, 85, 257–265.
- Ogawa, E.; Shimura, M.; Fushimi, T.; Tajika, M.; Ichimoto, K.; Matsunaga, A.; Tsuruoka, T.; Ishige, M.; Fuchigami, T.; Yamazaki, T.; et al. Clinical validity of biochemical and molecular analysis in diagnosing Leigh syndrome: A study of 106 Japanese patients. J. Inherit. Metab. Dis. 2017, 40, 685–693.
- Pek, N.M.Q.; Phua, Q.H.; Ho, B.X.; Pang, J.K.S.; Hor, J.-H.; An, O.; Yang, H.H.; Yu, Y.; Fan, Y.; Ng, S.-Y.; et al. Mitochondrial 3243A>G mutation confers pro-atherogenic and pro-inflammatory properties in MELAS iPS derived endothelial cells. Cell Death Dis. 2019, 10, 802.
- Kaufmann, P.; Engelstad, K.; Wei, Y.; Kulikova, R.; Oskoui, M.; Sproule, D.; Battista, V.; Koenigsberger, D.; Pascual, J.; Shanske, S.; et al. Natural history of MELAS associated with mitochondrial DNA m.3243A>G genotype. Neurology 2011, 77, 1965–1971.
- Seed, L.M.; Dean, A.; Krishnakumar, D.; Phyu, P.; Horvath, R.; Harijan, P.D. Molecular and neurological features of MELAS syndrome in paediatric patients: A case series and review of the literature. Mol. Genet. Genom. Med. 2022, 10, e1955.
- Al-Enezi, M.; Al-Saleh, H.; Nasser, M. Mitochondrial disorders with significant ophthalmic manifestations. Middle East Afr. J. Ophthalmol. 2008, 15, 81–86.
- Weiduschat, N.; Kaufmann, P.; Mao, X.; Engelstad, K.M.; Hinton, V.; DiMauro, S.; De Vivo, D.; Shungu, D. Cerebral metabolic abnormalities in A3243G mitochondrial DNA mutation carriers. Neurology 2014, 82, 798–805.
- El-Hattab, A.W.; Emrick, L.T.; Craigen, W.J.; Scaglia, F. Citrulline and arginine utility in treating nitric oxide deficiency in mitochondrial disorders. Mol. Genet. Metab. 2012, 107, 247–252.
- Yoshida, T.; Ouchi, A.; Miura, D.; Shimoji, K.; Kinjo, K.; Sueyoshi, T.; Jonosono, M.; Rajput, V. MELAS and reversible vasoconstriction of the major cerebral arteries. Intern. Med. 2013, 52, 1389–1392.
- Cheng, W.; Zhang, Y.; He, L. MRI Features of Stroke-Like Episodes in Mitochondrial Encephalomyopathy with Lactic Acidosis and Stroke-Like Episodes. Front. Neurol. 2022, 13, 843386.
- Ng, Y.S.; Bindoff, L.A.; Gorman, G.S.; Horvath, R.; Klopstock, T.; Mancuso, M.; Martikainen, M.H.; Mcfarland, R.; Nesbitt, V.; Pitceathly, R.D.S.; et al. Consensus-based statements for the management of mitochondrial stroke-like episodes. Wellcome Open Res. 2019, 4, 201.
- Argudo, J.M.; Moncayo, O.M.A.; Insuasti, W.; Garofalo, G.; Aguirre, A.S.; Encalada, S.; Villamarin, J.; Oña, S.; Tenemaza, M.G.; Eissa-Garcés, A.; et al. Arginine for the Treatment of Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-Like Episodes: A Systematic Review. Cureus 2022, 14, e32709.
- Pérez-Cruz, E.; González-Rivera, C.; Valencia-Olvera, L.d.C.G. Immunonutrition for the acute treatment of MELAS syndrome. Endocrinología. Diabetes Nutr. 2022, 69, 144–148.
- Li, J.; Zhang, W.; Cui, Z.; Li, Z.; Jiang, T.; Meng, H. Epilepsy Associated with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes. Front. Neurol. 2021, 12, 675816.
