Membrane separation technology has emerged as the preferred method for producing clean water during wastewater treatment and desalination. This preference is attributed to the high separation accuracy, energy efficiency, lack of secondary pollution, and ease of operation of the technology. Membrane fouling is a key obstacle in membrane applications, including ultrafiltration (UF), microfiltration (MF), nanofiltration (NF), and reverse osmosis (RO). Membrane fouling is a particularly serious problem in the pre-treatment processes of industrial wastewater, leading to poor water quality and increased operating costs. A thorough understanding of fouling formation and properties is required in wastewater treatment using membranes and contributes to slowing down membrane fouling and implementing appropriate control measures. In response, extensive foundational investigations of membrane fouling have been conducted, with researchers seeking to clarify primary foulants, membrane–foulant interactions, and potential fouling mitigation techniques.
- membrane fouling
- morphology
- roughness
- interactions
1. Characterization of Membrane
1.1. Characterization of Membrane Morphology
1.2. Characterization of Roughness
1.3. Measurement of Membrane Channels
2. Characterization of Contaminants
2.1. Organic Contaminants
2.2. Biological Contaminants
2.3. Emerging Contaminants
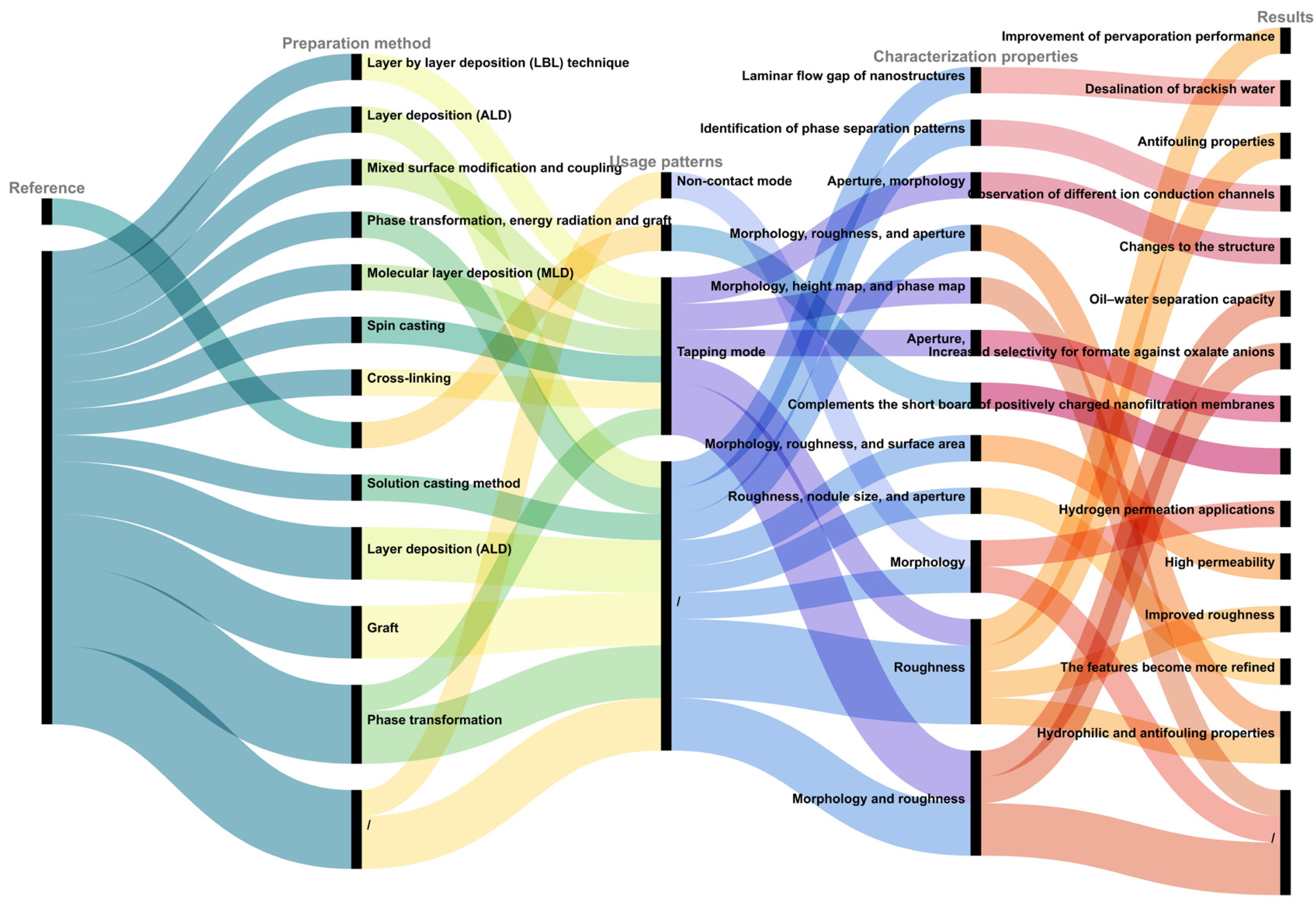
| Research Content | AFM Model | Characterization Properties | Results | Usage Patterns | Reference |
|---|---|---|---|---|---|
| Sodium alginate (SA) | Bruker AXS Multi-mode 8, Madison, WI, USA | Morphology | Alginates exist in single coiled chains | Contact mode | [30] |
| Effect of Na+ on organic fouling | Cypher ES, Oxford Instruments Asylum Research, Abingdon, UK | Morphology and interaction force | / | / | [49] |
| Effect of carboxyl and hydroxyl groups on adsorptive polysaccharide fouling | Cypher ES, Oxford Instruments Asylum Research, Abingdon, UK | Morphology | Transformation from ‘egg box’ model to formation of network gel | / | [50] |
| Effects of –COOH and –NH2 on adsorptive polysaccharide fouling | Cypher ES, Oxford Instruments Asylum Research, Abingdon, UK | Morphology and interaction force | In pH range 4–6, adherence of polysaccharide fouling and its reversibility depend on the functional groups |
Tapping mode | [51] |
| Effect of sodium and potassium on polysaccharide fouling on PVDF and graphene-oxide-modified PVDF membrane surfaces | Cypher ES, Oxford Instruments Asylum Research, Abingdon, UK | Interaction force | SA fouling in Na+ condition more severe than that in K+ owing to higher attraction forces under identical ion strengths | Tapping mode | [52] |
| Humic acid (HA) | Nanoscope IIIa SPM, Digital Instruments, Goleta, CA, USA | Morphology | Spherical particles and aggregates are found with apparent colloidal diameters < 100 nm and heights ranging from ~0.5 to ~7 nm | Tapping mode | [31] |
| Effect of Na+ and Mg2+ on adsorptive humic acid fouling | MultiMode 8.0 AFM (Bruker, Ettlingen, Germany) | Interaction force | Cations mainly affect HA fouling by controlling electrostatic and hydration forces of membrane–HA and HA–HA | Contact mode | [53] |
| Effect of Ca2+ and Mg2+ on adsorptive humic acid fouling | MultiMode 8.0 AFM (Bruker, Ettlingen, Germany) | Interaction force | Mitigation mechanisms differed for both ions | / | [54] |
| Bovine serum albumin (BSA) | / | Morphology | Most protein molecules are spread onto mica surface as monomers | Tapping mode | [32] |
| Effect of chlorination and ozonation on adsorptive protein fouling | MultiMode 8.0 atomic force microscope (AFM, Bruker, Ettlingen, Germany) | Interaction force | BSA fouling definitively mitigated by pre-chlorination but enhanced by pre-ozonation | Contact mode | [53] |
| Flagellar morphology of E. coli cultured at different pH conditions | Nanowizard AFM (JPK Instrument, Berlin, Germany) | Morphology | Differences in flagellar morphology at different pH values | Contact mode | [35] |
| E. coli under action of different disinfectants | Digital Instruments Veeco Metrology Group, Santa Barbara, CA, USA | Morphology | Differences in cell morphology under action of different disinfectants | Tapping mode | [36] |
| Changes in cell morphology of antibiotic-resistant E. coli | Asylum Research Cypher AFM (Oxford Instruments, Abingdon, UK) | Morphology | Damage to E. coli cells eventually leads to cell lysis | / | [37] |
| Different types of MPs | AFM diMultiMode V (Veeco, San Jose, CA, USA) | Morphology and roughness | Different types of MPs have different characteristics | / | [43] |
| Combined AFM and infrared spectroscopy IR (AFM-IR) characterization of MPs | / | Morphology and roughness | / | / | [44] |
| Forces between two NPs and E. coli | Agilent 5500 AFM (Molecular Imaging, Phoenix, AZ, USA) | Interaction force | Particle sizes of both hematite (α-FeO) and corundum (α-AlO) NPs significantly affected the strength of the adhesion force | Contact mode | [45] |
| Changes in hydrogel occurring when algae are present in the culture | HS-AFM, Bristol Nano Dynamics Ltd., Bristol, UK | Roughness | Roughness on the algal flocs significantly more pronounced than in the hydrogel layer | Contact mode | [38] |
| Clostridium perfringens treated by electrocoagulation floatation (ECF) method | AFM (Ntegra with Solaris platform, manufactured by NT MDT, Moscow, Russia) | Interaction force | Inefficiency of mechanical cell crushing process | Tapping mode | [39] |
3. Microscopic Identification of Membrane Fouling Processes under Changing Factors
4. Measurement of Interactions in Membrane Fouling
5. Modeling or Analysis of the Interaction in Membrane Fouling
References
- Stawikowska, J.; Livingston, A.G. Assessment of atomic force microscopy for characterisation of nanofiltration membranes. J. Membr. Sci. 2013, 425–426, 58–70.
- Wang, P.; Song, T.; Bu, J.; Zhang, Y.; Liu, J.; Zhao, J.; Zhang, T.; Xi, J.; Xu, J.; Li, L.; et al. Does bacterial community succession within the polyethylene mulching film plastisphere drive biodegradation? Sci. Total Environ. 2022, 824, 153884.
- He, Y.; Zhang, J.; Liang, X.; Shehzad, M.A.; Ge, X.; Zhu, Y.; Hu, M.; Yang, Z.; Wu, L.; Xu, T. Achieving high anion conductivity by densely grafting of ionic strings. J. Membr. Sci. 2018, 559, 35–41.
- Johnson, D.J.; Al Malek, S.A.; Al-Rashdi, B.A.M.; Hilal, N. Atomic force microscopy of nanofiltration membranes: Effect of imaging mode and environment. J. Membr. Sci. 2012, 389, 486–498.
- Olejnik, A.; Nowak, I. Atomic force microscopy analysis of synthetic membranes applied in release studies. Appl. Surf. Sci. 2015, 355, 686–697.
- San-Martín, M.I.; Carmona, F.J.; Alonso, R.M.; Prádanos, P.; Morán, A.; Escapa, A. Assessing the ageing process of cation exchange membranes in bioelectrochemical systems. Int. J. Hydrog. Energy 2019, 44, 25287–25296.
- Mulijani, S.; Mulanawati, A. Enhanced Performance of Asymmetric Polystyrene Membrane by Incorporation of Pluronic F127 and Its Application for Pervaporation Separation. Procedia Chem. 2012, 4, 360–366.
- Kumar, S.; Srivastava, S.; Vijay, Y.K. Study of gas transport properties of multi-walled carbon nanotubes/polystyrene composite membranes. Int. J. Hydrog. Energy 2012, 37, 3914–3921.
- Zafari, M.; Kikhavani, T.; Ashrafizadeh, S.N. Hybrid surface modification of an anion exchange membrane for selective separation of monovalent anions in the electrodialysis process. J. Environ. Chem. Eng. 2022, 10, 107104.
- Wu, C.; Zheng, J.; Hu, J. Novel antifouling polysulfone matrix membrane modified with zwitterionic polymer. J. Saudi Chem. Soc. 2021, 25, 101281.
- Ruangdit, S.; Chittrakarn, T.; Kaew-on, C.; Samran, R.; Bootluck, W.; Sirijarukul, S. E-beam induced grafting of binary monomer on polysulfone membrane for the separation of skim natural rubber latex. J. Environ. Chem. Eng. 2022, 10, 107862.
- Nie, Z.; Liu, C.; Jiang, X.; Zhou, Y.; Lin, X.; Zhao, X.; He, Q.; Chai, H.; Pang, X.; Ma, J. Dopamine-triggered one-step codeposition of zwitterionic surfactants for anti-fouling polyethersulfone ultrafiltration membrane modification. Appl. Surf. Sci. 2022, 598, 153871.
- Johnson, D.; Hilal, N. Polymer membranes—Fractal characteristics and determination of roughness scaling exponents. J. Membr. Sci. 2019, 570–571, 9–22.
- ElHadidy, A.M.; Peldszus, S.; Van Dyke, M.I. Development of a pore construction data analysis technique for investigating pore size distribution of ultrafiltration membranes by atomic force microscopy. J. Membr. Sci. 2013, 429, 373–383.
- Kim, T.N.; Lee, J.; Choi, J.H.; Ahn, J.H.; Yang, E.; Hwang, M.H.; Chae, K.J. Tunable atomic level surface functionalization of a multi-layered graphene oxide membrane to break the permeability-selectivity trade-off in salt removal of brackish water. Sep. Purif. Technol. 2021, 274, 119047.
- Mahmodi, G.; Ronte, A.; Dangwal, S.; Wagle, P.; Echeverria, E.; Sengupta, B.; Vatanpour, V.; McLlroy, D.N.; Ramsey, J.D.; Kim, S.-J. Improving antifouling property of alumina microfiltration membranes by using atomic layer deposition technique for produced water treatment. Desalination 2022, 523, 115400.
- Huang, A.; Kan, C.-C.; Lo, S.-C.; Chen, L.-H.; Su, D.-Y.; Soesanto, J.F.; Hsu, C.-C.; Tsai, F.-Y.; Tung, K.-L. Nanoarchitectured design of porous ZnO@copper membranes enabled by atomic-layer-deposition for oil/water separation. J. Membr. Sci. 2019, 582, 120–131.
- Welch, B.C.; McIntee, O.M.; Myers, T.J.; Greenberg, A.R.; Bright, V.M.; George, S.M. Molecular layer deposition for the fabrication of desalination membranes with tunable metrics. Desalination 2021, 520, 115334.
- Chandra, P.N.; Usha, K.; Mohan, M.K. Design, development and characterization of polyelectrolyte multilayer membranes for potential filtration applications. Mater. Today Proc. 2021, 41, 530–534.
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257.
- Ullah, A.; Tanudjaja, H.J.; Ouda, M.; Hasan, S.W.; Chew, J.W. Membrane fouling mitigation techniques for oily wastewater: A short review. J. Water Process Eng. 2021, 43, 102293.
- Meral, K.; Erbil, H.Y.; Onganer, Y. A spectroscopic study of water-soluble pyronin B and pyronin Y in Langmuir–Blodgett films mixed with stearic acid. Appl. Surf. Sci. 2011, 258, 1605–1612.
- Allen, F.I.; Ercius, P.; Modestino, M.A.; Segalman, R.A.; Balsara, N.P.; Minor, A.M. Deciphering the three-dimensional morphology of free-standing block copolymer thin films by transmission electron microscopy. Micron 2013, 44, 442–450.
- Chakraborty, S.; Wang, B.; Dutta, P.K. Tolerance of polymer-zeolite composite membranes to mechanical strain. J. Membr. Sci. 2016, 518, 192–202.
- Llanos, J.; Williams, P.M.; Cheng, S.; Rogers, D.; Wright, C.; Perez, A.; Canizares, P. Characterization of a ceramic ultrafiltration membrane in different operational states after its use in a heavy-metal ion removal process. Water Res. 2010, 44, 3522–3530.
- Cheng, S.; Oatley, D.L.; Williams, P.M.; Wright, C.J. Positively charged nanofiltration membranes: Review of current fabrication methods and introduction of a novel approach. Adv. Colloid Interface Sci. 2011, 164, 12–20.
- Ni, T.; You, Y.; Xie, Z.; Kong, L.; Newman, B.; Henderson, L.; Zhao, S. Waste-derived carbon fiber membrane with hierarchical structures for enhanced oil-in-water emulsion separation: Performance and mechanisms. J. Membr. Sci. 2022, 653, 120543.
- Jafari, B.; Rezaei, E.; Dianat, M.J.; Abbasi, M.; Hashemifard, S.A.; Khosravi, A.; Sillanpää, M. Development of a new composite ceramic membrane from mullite, silicon carbide and activated carbon for treating greywater. Ceram. Int. 2021, 47, 34667–34675.
- Teng, J.; Wu, M.; Chen, J.; Lin, H.; He, Y. Different fouling propensities of loosely and tightly bound extracellular polymeric substances (EPSs) and the related fouling mechanisms in a membrane bioreactor. Chemosphere 2020, 255, 126953.
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation behavior and mechanism of alginate with calcium: Dependence on monovalent counterions. Carbohydr. Polym. 2022, 294, 119788.
- Chen, C.; Wang, X.; Jiang, H.; Hu, W. Direct observation of macromolecular structures of humic acid by AFM and SEM. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 121–125.
- Demaneche, S.; Chapel, J.P.; Monrozier, L.J.; Quiquampoix, H. Dissimilar pH-dependent adsorption features of bovine serum albumin and alpha-chymotrypsin on mica probed by AFM. Colloids Surf. B Biointerfaces 2009, 70, 226–231.
- Gao, K.; Li, T.; Zhao, Q.; Liu, W.; Liu, J.; Song, Y.; Chu, H.; Dong, B. UF fouling behavior of allelopathy of extracellular organic matter produced by mixed algae co-cultures. Sep. Purif. Technol. 2021, 261, 118297.
- Song, W. Nanofiltration of natural organic matter with H2O2/UV pretreatment: Fouling mitigation and membrane surface characterization. J. Membr. Sci. 2004, 241, 143–160.
- Chang, K.C.; Cheng, S.J.; Chen, Y.C.; Huang, H.R.; Liou, J.W. Nanoscopic analysis on pH induced morphological changes of flagella in Escherichia coli. J. Microbiol. Immunol. Infect. 2013, 46, 405–412.
- Zorila, F.L.; Ionescu, C.; Craciun, L.S.; Zorila, B. Atomic force microscopy study of morphological modifications induced by different decontamination treatments on Escherichia coli. Ultramicroscopy 2017, 182, 226–232.
- Ahmed, Y.; Zhong, J.; Yuan, Z.; Guo, J. Simultaneous removal of antibiotic resistant bacteria, antibiotic resistance genes, and micropollutants by a modified photo-Fenton process. Water Res. 2021, 197, 117075.
- Landels, A.; Beacham, T.A.; Evans, C.T.; Carnovale, G.; Raikova, S.; Cole, I.S.; Goddard, P.; Chuck, C.; Allen, M.J. Improving electrocoagulation floatation for harvesting microalgae. Algal Res. 2019, 39, 101446.
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Force and energy requirement for microalgal cell disruption: An atomic force microscope evaluation. Bioresour. Technol. 2013, 128, 199–206.
- Li, M. Chapter 7—Nanoscale imaging and force probing of single microbial cells by atomic force microscopy. In Atomic Force Microscopy for Nanoscale Biophysics; Li, M., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 187–217.
- Wang, Y.; Zheng, X.; Xiao, K.; Xue, J.; Ulbricht, M.; Zhang, Y. How and why does time matter—A comparison of fouling caused by organic substances on membranes over adsorption durations. Sci. Total Environ. 2023, 866, 160655.
- Cai, L.; Wu, D.; Xia, J.; Shi, H.; Kim, H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci. Total Environ. 2019, 671, 1101–1107.
- Melo-Agustin, P.; Kozak, E.R.; de Jesus Perea-Flores, M.; Mendoza-Perez, J.A. Identification of microplastics and associated contaminants using ultra high resolution microscopic and spectroscopic techniques. Sci. Total Environ. 2022, 828, 154434.
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19.
- Zhang, W.; Stack, A.G.; Chen, Y. Interaction force measurement between E. coli cells and nanoparticles immobilized surfaces by using AFM. Colloids Surf. B Biointerfaces 2011, 82, 316–324.
- Liu, Y.; Yuan, S.; Chi, M.; Wang, Y.; Van Eygen, G.; Zhao, R.; Zhang, X.; Li, G.; Volodine, A.; Hu, S.; et al. Efficient capture of endocrine-disrupting compounds by a high-performance nanofiltration membrane for wastewater treatment. Water Res. 2022, 227, 119322.
- Wu, J.; Lu, L.; Wang, R.; Pan, L.; Chen, B.; Zhu, X. Influence of microplastics on the transport of antibiotics in sand filtration investigated by AFM force spectroscopy. Sci. Total Environ. 2023, 873, 162344.
- Wu, J.; Wang, R.; Zhang, Y.; Chen, B.; Zhu, X. In situ scrutinize the adsorption of sulfamethoxazole in water using AFM force spectroscopy: Molecular adhesion force determination and fractionation. J. Hazard. Mater. 2022, 426, 128128.
- Zhai, Y.; Bai, D.; Wang, Y.; Zhang, Y.; Qi, Y.; Qiu, X.; Wang, Y.-f.; Wang, Y.X.; Zheng, X. Effect of Na+ on organic fouling depends on Na+ concentration and the property of the foulants. Desalination 2022, 531, 115709.
- Zhang, Y.; Wang, Y.; Cao, X.; Xue, J.; Zhang, Q.; Tian, J.; Li, X.; Qiu, X.; Pan, B.; Gu, A.Z.; et al. Effect of carboxyl and hydroxyl groups on adsorptive polysaccharide fouling: A comparative study based on PVDF and graphene oxide (GO) modified PVDF surfaces. J. Membr. Sci. 2020, 595, 117514.
- Wang, Y.; Zheng, X.; Wang, Z.; Shi, Z.; Kong, Z.; Zhong, M.; Xue, J.; Zhang, Y. Effects of –COOH and –NH2 on adsorptive polysaccharide fouling under varying pH conditions: Contributing factors and underlying mechanisms. J. Membr. Sci. 2021, 621, 118933.
- Wang, Y.; Zheng, X.; Li, D.; Meng, F.; Tian, J.; Wang, M.; Li, L.; Wu, H.; Zhang, Y. Effect of sodium and potassium on polysaccharide fouling on PVDF and graphene oxide modified PVDF membrane surfaces. Process Saf. Environ. Prot. 2022, 165, 387–395.
- Miao, R.; Zhou, Y.; Wang, P.; Lu, W.; Li, P.; Li, X.; Wang, L. A comparison of effect mechanisms of chlorination and ozonation on the interfacial forces of protein at membrane surfaces and the implications for membrane fouling control. J. Membr. Sci. 2021, 628, 119266.
- Zhang, Y.; Wang, T.; Meng, J.; Lei, J.; Zheng, X.; Wang, Y.; Zhang, J.; Cao, X.; Li, X.; Qiu, X.; et al. A novel conductive composite membrane with polypyrrole (PPy) and stainless-steel mesh: Fabrication, performance, and anti-fouling mechanism. J. Membr. Sci. 2020, 621, 118937.
- Miao, R.; Wang, L.; Deng, D.; Li, S.; Wang, J.; Liu, T.; Zhu, M.; Lv, Y. Evaluating the effects of sodium and magnesium on the interaction processes of humic acid and ultrafiltration membrane surfaces. J. Membr. Sci. 2017, 526, 131–137.
- Miao, R.; Li, X.; Wu, Y.; Wang, P.; Wang, L.; Wu, G.; Wang, J.; Lv, Y.; Liu, T. A comparison of the roles of Ca2+ and Mg2+ on membrane fouling with humic acid: Are there any differences or similarities? J. Membr. Sci. 2018, 545, 81–87.
- Arkhangelsky, E.; Bazarbayeva, A.; Kamal, A.; Kim, J.; Inglezakis, V.; Gitis, V. Tangential streaming potential, transmembrane flux, and chemical cleaning of ultrafiltration membranes. Sep. Purif. Technol. 2021, 258, 118045.
- Hashino, M.; Hirami, K.; Ishigami, T.; Ohmukai, Y.; Maruyama, T.; Kubota, N.; Matsuyama, H. Effect of kinds of membrane materials on membrane fouling with BSA. J. Membr. Sci. 2011, 384, 157–165.
- Meng, X.R.; Tang, W.T.; Wang, L.; Wang, X.D.; Huang, D.X.; Chen, H.N.; Zhang, N. Mechanism analysis of membrane fouling behavior by humic acid using atomic force microscopy: Effect of solution pH and hydrophilicity of PVDF ultrafiltration membrane interface. J. Membr. Sci. 2015, 487, 180–188.
- Heffernan, R.; Habimana, O.; Semiao, A.J.; Cao, H.; Safari, A.; Casey, E. A physical impact of organic fouling layers on bacterial adhesion during nanofiltration. Water Res. 2014, 67, 118–128.
- Gao, Z.; Mi, N.; Liu, T. Preparation of a polyvinylidene fluoride membrane material probe and its application in membrane fouling research. Desalination 2015, 357, 171–177.
- Miao, R.; Wang, L.; Lv, Y.; Wang, X.; Feng, L.; Liu, Z.; Huang, D.; Yang, Y. Identifying polyvinylidene fluoride ultrafiltration membrane fouling behavior of different effluent organic matter fractions using colloidal probes. Water Res. 2014, 55, 313–322.
- Wang, Y.; Zheng, X.; Li, D.; Tian, J.; Wu, H.; Zhang, Y. Comparison of membrane fouling induced by protein, polysaccharide and humic acid under sodium and calcium ionic conditions. Desalination 2023, 548, 116236.
- Fu, W.; Wang, L.; Chen, F.; Zhang, X.; Zhang, W. Polyvinyl chloride (PVC) ultrafiltration membrane fouling and defouling behavior: EDLVO theory and interface adhesion force analysis. J. Membr. Sci. 2018, 564, 204–210.
- Mozia, S.; Darowna, D.; Orecki, A.; Wróbel, R.; Wilpiszewska, K.; Morawski, A.W. Microscopic studies on TiO2 fouling of MF/UF polyethersulfone membranes in a photocatalytic membrane reactor. J. Membr. Sci. 2014, 470, 356–368.
- Guo, H.; Xiao, L.; Yu, S.; Yang, H.; Hu, J.; Liu, G.; Tang, Y. Analysis of anion exchange membrane fouling mechanism caused by anion polyacrylamide in electrodialysis. Desalination 2014, 346, 46–53.
- Englert, A.H.; Ren, S.; Masliyah, J.H.; Xu, Z. Interaction forces between a deformable air bubble and a spherical particle of tuneable hydrophobicity and surface charge in aqueous solutions. J. Colloid Interface Sci. 2012, 379, 121–129.
- Zhang, S.; Gutierrez, L.; Niu, X.Z.; Qi, F.; Croue, J.P. The characteristics of organic matter influence its interfacial interactions with MnO(2) and catalytic oxidation processes. Chemosphere 2018, 209, 950–959.
- Villacorte, L.O.; Ekowati, Y.; Neu, T.R.; Kleijn, J.M.; Winters, H.; Amy, G.; Schippers, J.C.; Kennedy, M.D. Characterisation of algal organic matter produced by bloom-forming marine and freshwater algae. Water Res. 2015, 73, 216–230.
- Yumiyama, S.; Kato, S.; Konishi, Y.; Nomura, T. Direct measurement of interaction forces between a yeast cell and a microbubble using atomic force microscopy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123963.
- Wu, J.; Contreras, A.E.; Li, Q. Studying the impact of RO membrane surface functional groups on alginate fouling in seawater desalination. J. Membr. Sci. 2014, 458, 120–127.
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143.
- Brant, J.A.; Childress, A.E. Assessing short-range membrane–colloid interactions using surface energetics. J. Membr. Sci. 2002, 203, 257–273.
- Zhenga, Y.; Zhanga, W.; Tanga, B.; Bina, L.; Dinga, J. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2018, 250, 398.
- Gomes, M.C.S.; Moreira, W.M.; Paschoal, S.M.; Sipoli, C.C.; Suzuki, R.M.; Sgorlon, J.G.; Pereira, N.C. Modeling Of Fouling Mechanisms In The Biodiesel Purification Using Ceramic Membranes. Sep. Purif. Technol. 2021, 269, 118595.
- Lin, T.; Lu, Z.J.; Chen, W. Interaction mechanisms and predictions on membrane fouling in an ultrafiltration system, using the XDLVO approach. J. Membr. Sci. 2014, 461, 49–58.
- Xia, T.; Li, S.; Wang, H.; Guo, C.; Liu, C.; Liu, A.; Guo, X.; Zhu, L. Insights into the Transport of Pristine and Photoaged Graphene Oxide-Hematite Nanohybrids in Saturated Porous Media: Impacts of XDLVO Interactions and Surface Roughness. J. Hazard. Mater. 2021, 419, 126488.
- Li, R.; Lou, Y.; Xu, Y.; Ma, G.; Liao, B.Q.; Shen, L.; Lin, H. Effects of surface morphology on alginate adhesion: Molecular insights into membrane fouling based on XDLVO and DFT analysis. Chemosphere 2019, 233, 373–380.
- Ou, Q.; Xu, Y.; Li, X.; He, Q.; Liu, C.; Zhou, X.; Wu, Z.; Huang, R.; Song, J.; Huangfu, X. Interactions between activated sludge extracellular polymeric substances and model carrier surfaces in WWTPs: A combination of QCM-D, AFM and XDLVO prediction. Chemosphere 2020, 253, 126720.
- Shee Keat, M. Membrane Technology for Glycerin Purification. Doctoral Dissertation, Monash University, Faculty of Engineering, Chemical Engineering, Clayton, Malaysia, 2017. Available online: https://bridges.monash.edu/articles/thesis/Membrane_technology_for_glycerin_purification/4652773 (accessed on 1 May 2023).
- Huang, H.; Young, T.A.; Jacangelo, J.G. Unified Membrane Fouling Index for Low Pressure Membrane Filtration of Natural Waters: Principles and Methodology. Environ. Sci. Technol. 2008, 42, 714.
