Acute respiratory failure (ARF) is a challenging condition that clinicians, especially in emergency settings, have to face frequently. Especially in emergency settings, many underlying diseases can lead to ARF and life-threatening conditions have to be promptly assessed and correctly treated to avoid unfavorable outcomes. Point-of-care ultrasound (POCUS) gained growing consideration due to its bedside utilization, reliability and reproducibility even in emergency settings especially in unstable patients. This is a narrative review on the use of POCUS to assess airway and breathing impairments in patients presenting ARF.
- acute respiratory failure
- lung ultrasound
- upper airways management
- pneumonia
- pneumothorax
- lung effusion
1. Introduction
2. The Role of Pocus in Acute Respiratory Failure
2.1. A: US & Airway
2.1.1. Endotracheal Tube (ETT) Positioning Assessment
2.1.2. Upper Airways Damage Identification and Procedures
US visualization of upper airway structures and their abnormalities is a recent role of the POCUS application. Cases of laryngeal ACE-I-induced larynx edema [11][9] and trauma affecting the larynx have been approached with US to assess the extent of damage [12][10]. An important finding in upper airways US is cricoid membrane identification. US has been extensively used to guide invasive procedures and its role to support cricothyrotomy has been evaluated. In a prospective observational study emergency physicians applied a technique first learned in a cadaver laboratory and then applied in vivo. US did not affect the time of execution, as the mean time required was 24.32 s. This time was not affected by patient anatomy or body mass index (BMI) [13][11]. There are some case reports about patients presenting with a critical mass in the larynx. Upper airways US permitted to evaluate their extension and to identify the feasibility of cricothyroidotomy instead of emergency tracheostomy. In one of these cases, US was also applied to visualize the hyoid bone to assess short hyomental distance ratio, high pre-tracheal anterior neck thickness and tongue size to predict endotracheal tube size [14,15][12][13].2.1.3. Laryngeal Edema Assessment Pre-Extubation
The assessment of the larynx is important to predict extubation failure. Usually, this is evaluated by a leak test (difference between expiratory tidal volumes with the cuff inflated and deflated). Two prospective observational studies evaluated the air column width differences (ACWD) (width of air between the vocal cords seen by laryngeal ultrasonography) as a predictive index of extubation failure. In the first study ACWD ≥ 1.6 mm predicted laryngeal edema with 0.706 and 0.702 sensitivity and specificity, respectively; the area under the receiver operating characteristic curve of laryngeal ultrasound was 0.823 (95% confidence interval, 0.698–0.947) and that of cuff leak test was 0.840 [19][14]. In the other study, both laryngeal US and leak test resulted in having a positive predictive value < 20% cuff leak test (cut-off point: 249 mL) and showed a sensitivity and specificity of 75% and 59%, respectively.2.2. B: US and Breathing
2.2.1. Protocols on Lung US
The BLUE protocol (Figure 41) is a flow chart to approach acute respiratory failure and its differential diagnosis by lung US (LUS) in a standardized way proposed by Lichtenstein and updated in 2008 [23][15].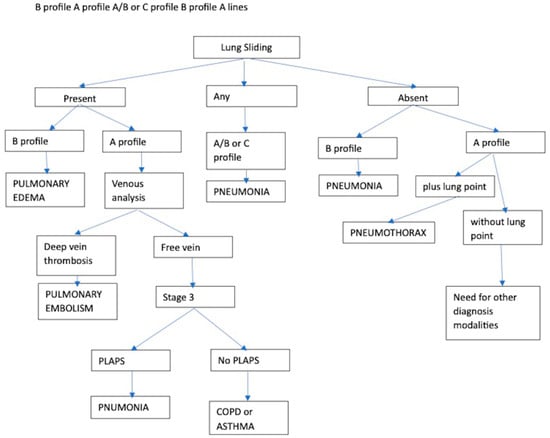
- -
-
Abolition of lung sliding alone, sensitivity 100% specificity 78%;
- -
-
Absent lung sliding plus the A-line sign, sensitivity 95% specificity 94%;
- -
-
Lung point, sensitivity 79% specificity 100% [35][25].
Acute Respiratory Distress Syndrome (ARDS)
A particular mention to ARDS, US has been investigated for diagnostic and monitorization purposes, as it can predict CT findings about lung aeration, monitor lung re-aeration during treatment and identify tidal volume recruitment [84][29]. Many prospective observational studies evaluated lung ultrasound scores in ARDS. Firstly, the combination of LUS plus pulse oximetry showed a better diagnostic accuracy than chest radiography plus blood gas analysis [39][30]. Moreover, LUS correlated well with oxygenation (P/F ratio) and seems to have a prognostic value about survival in mechanically ventilated patients and post-extubation distress syndrome [40,41,42,43][31][32][33][34]. LUS application in ARDS assessment has been so extensively studied that a possible correlation with a genetic polymorphism in the plasma platelet-activating factor was published. G994T polymorphism, combined with LUS score, showed a negative correlation with respiratory failure index, the need for ventilation, lactic acid levels, SOFA score, etc. A combination of LUS score and G994T polymorphism may be employed as a potential prognostic marker for ARDS [45][35] (Figure 52).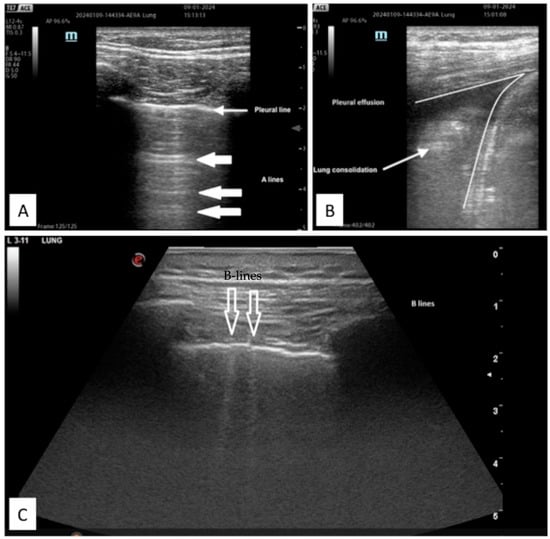 Figure 52. Example of LUS images of (A) normal aerated lung with pleural and A-line; (B) pleural effusion and associated parenchymal consolidation; (C) B-lines sign of interstitial syndrome.
Figure 52. Example of LUS images of (A) normal aerated lung with pleural and A-line; (B) pleural effusion and associated parenchymal consolidation; (C) B-lines sign of interstitial syndrome.2.2.2. Diagnostic Accuracy
In this prospective observational study conducted by Barman et al., POCUS was performed after a first clinical diagnosis was made. Out of 108 enrolled patients, initial clinical diagnosis was appropriate in 67.5% of cases, after POCUS assessment the diagnostic accuracy raised to 88% adding or changing the diagnosis in 37% of cases. Similar improvements were observed in the treatment plan decided before and after POCUS, in 36% of cases treatment decisions were changed. This study highlights how POCUS can improve diagnostic accuracy and lead to different treatment choices in clinical practice [50][36].
Furthermore, in this interventional study by Sen et al. about the medical emergency team activities, POCUS was proved to be feasible and reliable for in-hospital emergency management [51][37].Diagnostic accuracy has also been evaluated for single pathology. The accuracy of LUS in a prospective observational study [52][38] is reported below:- -
-
Pneumonia, standard, 0.74, ultrasound, 0.87;
- -
-
Acute hemodynamic pulmonary edema standard, 0.79, ultrasound, 0.93);
- -
-
Decompensated COPD standard, 0.8, ultrasound, 0.92;
- -
-
Pulmonary embolism standard, 0.65, ultrasound, 0.81;Two meta-analyses [53,54][39][40] report sensitivity and specificity for the following diagnosis comparing the standard of care to US:
- -
-
Pneumonia/consolidation 89–92% and 94–97%;
- -
-
Heart failure/interstitial syndrome 90–95% and 91–93%;
- -
-
Pleural effusion 95% and 99%;
- -
-
COPD/asthma (A profile) 78% and 94%.POCUS diagnostic accuracy has also been assessed comparing specifically LUS to CT scan findings in ARF patients. Overall, LUS sensitivity and specificity were 82.7–92.3% and 90.2–98.6% reaching a global agreement with CT scans ranging from 0.640 (0.391–0.889) to 0.934 (0.605–1.000) with an average of 0.775 (0.577–0.973) [53][39].
2.2.3. Time-to-Diagnosis Improvement
POCUS turned out to be useful also in shortening the time to reach a diagnosis and reducing patient overall management. One of Lichtenstein’s works on US is reported to save up to two hours for diagnosis and management [23][15]. The mean time for diagnosis was shorter in POCUS application versus standard care. In these two prospective observational studies and a prospective randomized study, the time needed for a diagnosis was 12–42 min with POCUS, against 79–270 min with usual clinical care [59,60,61][41][42][43].2.2.4. Diaphragm Ultra-Sound (DUS)
Other than lung ultrasound, the evaluation of the diaphragm in ARF has been studied to assess the entity of respiratory distress. Even if DUS is not precisely standardized yet [85][44], with US is possible to obtain information about diaphragm movement. As the diaphragm is the most important inspiratory muscle, its dysfunction has a great impact on the deterioration of respiratory function. Indeed, literature about DUS has been focused on its predictive value: prediction of respiratory failure, NIV failure and weaning/extubation failure [62][45]. To quantify diaphragm movement by DUS clinicians can measure (see Figure 63):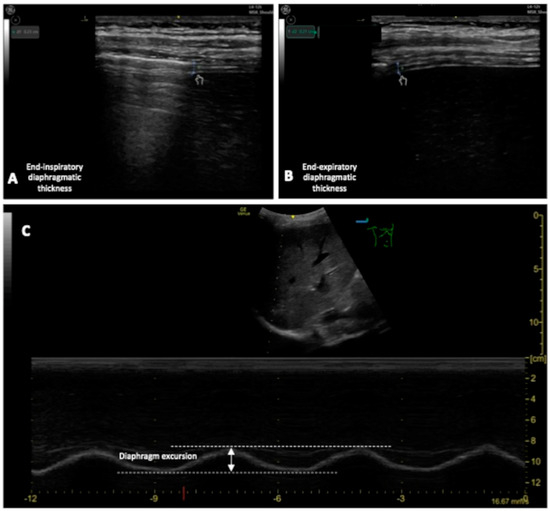 Figure 63.(A,B): Measurement of the end-inspiratory and end-expiratory diaphragmatic thickness; (C) measurement of the DE.
Figure 63.(A,B): Measurement of the end-inspiratory and end-expiratory diaphragmatic thickness; (C) measurement of the DE.-
Diaphragm thickening fraction (DTF), measurement of the difference in end-inspiratory and end-expiratory diaphragmatic thickness, expressed as a fraction;
3. Conclusions
POCUS application to assess ARF is becoming a useful and reliable tool, especially in emergency settings supported by growing scientific evidence. The availability of an ultrasound machine in increasing settings allows its application in many different clinical conditions, thus its utilization should be implemented and reported to increase literature evidence on its potentiality. Emergency medicine is one of the main disciplines where POCUS may make the difference between life and death being useful also in procedural intervention guidance if needed. Individual competence, poor resources and adverse environmental conditions may limit its application; however, updated and new ultrasound technology may help clinicians to fill these gaps. Unfortunately, the grade of scientific evidence on POCUS such as clinical trials is poor, and its increasing utilization should lead to conducting studies with a stronger level of evidence such as clinical trials.
References
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693.
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. LUNG SAFE Investigators, & ESICM Trials Group Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800.
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740.
- Williams, J.P.; Nathanson, R.; LoPresti, C.M.; Mader, M.J.; Haro, E.K.; Drum, B.; O’Brien, E.; Khosla, R.; Boyd, J.S.; Bales, B.; et al. Current use, training, and barriers in point-of-care ultrasound in hospital medicine: A national survey of VA hospitals. J. Hosp. Med. 2022, 17, 601–608.
- Abrokwa, S.K.; Ruby, L.C.; Heuvelings, C.C.; Bélard, S. Task shifting for point of care ultrasound in primary healthcare in low- and middle-income countries—A systematic review. EClinicalMedicine 2022, 45, 101333.
- Kreiser, M.A.; Hill, B.; Karki, D.; Wood, E.; Shelton, R.; Peterson, J.; Riccio, J.; Zapata, I.; Khalil, P.A.; Gubler, D.; et al. Point-of-Care Ultrasound Use by EMS Providers in Out-of-Hospital Cardiac Arrest. Prehosp. Disaster Med. 2022, 37, 39–44.
- Hossein-Nejad, H.; Mehrjerdi, M.S.; Abdollahi, A.; Loesche, M.A.; Schulwolf, S.; Ghadipasha, M.; Mohammadinejad, P.; Ataeinia, B.; Shokoohi, H. Ultrasound for Intubation Confirmation: A Randomized Controlled Study among Emergency Medicine Residents. Ultrasound Med. Biol. 2021, 47, 230–235.
- Chenkin, J.; McCartney, C.J.; Jelic, T.; Romano, M.; Heslop, C.; Bandiera, G. Defining the learning curve of point-of-care ultrasound for confirming endotracheal tube placement by emergency physicians. Crit. Ultrasound J. 2015, 7, 14.
- Schick, M.; Grether-Jones, K. Point-of-Care Sonographic Findings in Acute Upper Airway Edema. West. J. Emerg. Med. 2016, 17, 822–826.
- Adi, O.; Sum, K.M.; Ahmad, A.H.; Wahab, M.A.; Neri, L.; Panebianco, N. Novel role of focused airway ultrasound in early airway assessment of suspected laryngeal trauma. Ultrasound J. 2020, 12, 37.
- Nicholls, S.E.; Sweeney, T.W.; Ferre, R.M.; Strout, T.D. Bedside sonography by emergency physicians for the rapid identification of landmarks relevant to cricothyrotomy. Am. J. Emerg. Med. 2008, 26, 852–856.
- Adi, O.; Fong, C.P.; Sum, K.M.; Ahmad, A.H. Usage of airway ultrasound as an assessment and prediction tool of a difficult airway management. Am. J. Emerg. Med. 2021, 42, 263.e1–263.e4.
- Iqhbal, M.; Noor, J.M.; Karim, N.A.; Ismail, I.; Sanib, H.; Mokhtar, M.A.; Salim, S.S.F. Point-of-Care Airway Ultrasonography Prior to an Emergency Cricothyroidotomy: Case Report. Sultan Qaboos Univ. Med. J. 2018, 18, e219–e222.
- Wong, K.P.; Au, K.P.; Lam, S.; Lang, B.H. Lessons Learned After 1000 Cases of Transcutaneous Laryngeal Ultrasound (TLUSG) with Laryngoscopic Validation: Is There a Role of TLUSG in Patients Indicated for Laryngoscopic Examination Before Thyroidectomy? Thyroid Off. J. Am. Thyroid Assoc. 2017, 27, 88–94.
- Lichtenstein, D.A.; Mezière, G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008, 134, 117–125.
- Lichtenstein, D. Lung ultrasound in the critically ill. Curr. Opin. Crit. Care 2014, 20, 315–322.
- Lichtenstein, D. Novel approaches to ultrasonography of the lung and pleural space: Where are we now? Breathe 2017, 13, 100–111.
- Neto, F.L.D.; De Andrade, J.M.S.; Raupp, A.C.T.; Townsend, R.D.S.; Beltrami, F.G.; Brisson, H.; Lu, Q.; Dalcin, P.D.T.R. Diagnostic accuracy of the Bedside Lung Ultrasound in Emergency protocol for the diagnosis of acute respiratory failure in spontaneously breathing patients. J. Bras. Pneumol. 2015, 41, 58–64.
- Patel, C.J.; Bhatt, H.B.; Parikh, S.N.; Jhaveri, B.N.; Puranik, J.H. Bedside Lung Ultrasound in Emergency Protocol as a Diagnostic Tool in Patients of Acute Respiratory Distress Presenting to Emergency Department. J. Emerg. Trauma Shock 2018, 11, 125–129.
- Arslan, B.; Sonmez, O. Diagnosis of a Ruptured Pulmonary Hydatid Cyst in a 26-Week Pregnant Female with Bedside Lung Ultrasound in Emergency (BLUE) Protocol: A Case Report. Cureus 2022, 14, e25431.
- Haaksma, M.E.; Nossent, E.J.; Elbers, P.; Tuinman, P.R. Case Report: Pulmonary hemorrhage as a rare cause of lung ultrasound A/B-profile. F1000Research 2019, 8, 788.
- Lichtenstein, D.; van Hooland, S.; Elbers, P.; Malbrain, M.L. Ten good reasons to practice ultrasound in critical care. Anaesthesiol. Intensive Ther. 2014, 46, 323–335.
- Staub, L.J.; Mazzali Biscaro, R.R.; Kaszubowski, E.; Maurici, R. Lung Ultrasound for the Emergency Diagnosis of Pneumonia, Acute Heart Failure, and Exacerbations of Chronic Obstructive Pulmonary Disease/Asthma in Adults: A Systematic Review and Meta-analysis. J. Emerg. Med. 2019, 56, 53–69.
- Reissig, A.; Copetti, R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration 2014, 87, 179–189.
- Lichtenstein, D.A.; Mezière, G.; Lascols, N.; Biderman, P.; Courret, J.P.; Gepner, A.; Goldstein, I.; Tenoudji-Cohen, M. Ultrasound diagnosis of occult pneumothorax. Crit. Care Med. 2005, 33, 1231–1238.
- Zhang, G.; Huang, X.Y.; Zhang, L. Ultrasound guiding the rapid diagnosis and treatment of perioperative pneumothorax: A case report. World J. Clin. Cases 2021, 9, 11043–11049.
- Mallow, C.; Isakow, W. Risk Factors for Loss of Lung Sliding in a Medical Intensive Care Population with Acute Respiratory Failure. J. Bronchol. Interv. Pulmonol. 2019, 26, 102–107.
- Aziz, S.G.; Patel, B.B.; Ie, S.R.; Rubio, E.R. The Lung Point Sign, not Pathognomonic of a Pneumothorax. Ultrasound Q. 2016, 32, 277–279.
- Cereda, M.; Xin, Y.; Goffi, A.; Herrmann, J.; Kaczka, D.W.; Kavanagh, B.P.; Perchiazzi, G.; Yoshida, T.; Rizi, R.R. Imaging the Injured Lung: Mechanisms of Action and Clinical Use. Anesthesiology 2019, 131, 716–749.
- Bass, C.M.; Sajed, D.R.; Adedipe, A.A.; West, T.E. Pulmonary ultrasound and pulse oximetry versus chest radiography and arterial blood gas analysis for the diagnosis of acute respiratory distress syndrome: A pilot study. Crit. Care 2015, 19, 282.
- Todur, P.; Souvik Chaudhuri FNB Critical Care; Vedaghosh Amara FNB Critical Care; Srikant, N.; Prakash, P. Correlation of Oxygenation and Radiographic Assessment of Lung Edema (RALE) Score to Lung Ultrasound Score (LUS) in Acute Respiratory Distress Syndrome (ARDS) Patients in the Intensive Care Unit. Can. J. Respir. Ther. 2021, 57, 53–59.
- Zhao, Z.; Jiang, L.; Xi, X.; Jiang, Q.; Zhu, B.; Wang, M.; Xing, J.; Zhang, D. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm. Med. 2015, 15, 98.
- Xie, Y.; Liu, S.; Mou, Z.; Wang, Y.; Li, X. Correlation Analysis between Mechanical Power and Lung Ultrasound Score and Their Evaluation of Severity and Prognosis in ARDS Patients. BioMed Res. Int. 2021, 2021, 4156162.
- Wang, R.; Qi, B.; Zhang, X.; Meng, L.; Wu, X. Prophetic values of lung ultrasound score on post-extubation distress in patients with acute respiratory distress syndrome. Eur. J. Med. Res. 2022, 27, 27.
- Lv, W.; Wang, S.; Wang, L.; Wu, Z.; Jiang, Y.; Chen, X.; Gao, R. G994T polymorphism in exon 9 of plasma platelet-activating factor acetylhydrolase gene and lung ultrasound score as prognostic markers in evaluating the outcome of acute respiratory distress syndrome. Exp. Ther. Med. 2019, 17, 3174–3180.
- Barman, B.; Parihar, A.; Kohli, N.; Agarwal, A.; Dwivedi, D.K.; Kumari, G. Impact of Bedside Combined Cardiopulmonary Ultrasound on Etiological Diagnosis and Treatment of Acute Respiratory Failure in Critically Ill Patients. Indian J. Crit. Care Med. 2020, 24, 1062–1070.
- Sen, S.; Acash, G.; Sarwar, A.; Lei, Y.; Dargin, J.M. Utility and diagnostic accuracy of bedside lung ultrasonography during medical emergency team (MET) activations for respiratory deterioration. J. Crit. Care 2017, 40, 58–62.
- Silva, S.; Biendel, C.; Ruiz, J.; Olivier, M.; Bataille, B.; Geeraerts, T.; Mari, A.; Riu, B.; Fourcade, O.; Genestal, M. Usefulness of cardiothoracic chest ultrasound in the management of acute respiratory failure in critical care practice. Chest 2013, 144, 859–865.
- Yuan, X.; Liu, L.; Chang, W.; Wu, Z.; Huang, L.; Chao, Y.; Lu, X.; Xie, J.; Yang, Y.; Qiu, H. Diagnosis Accuracy of Lung Ultrasound for ARF in Critically Ill Patients: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 705960.
- Smit, J.M.; Haaksma, M.E.; Winkler, M.H.; Heldeweg, M.L.A.; Arts, L.; Lust, E.J.; Elbers, P.W.G.; Meijboom, L.J.; Girbes, A.R.J.; Heunks, L.M.A.; et al. Lung ultrasound in a tertiary intensive care unit population: A diagnostic accuracy study. Crit. Care 2021, 25, 339.
- Gaber, H.R.; Mahmoud, M.I.; Carnell, J.; Rohra, A.; Wuhantu, J.; Williams, S.; Rafique, Z.; Peacock, W.F. Diagnostic accuracy and temporal impact of ultrasound in patients with dyspnea admitted to the emergency department. Clin. Exp. Emerg. Med. 2019, 6, 226–234.
- Zare, M.A.; Bahmani, A.; Fathi, M.; Arefi, M.; Hossein Sarbazi, A.; Teimoori, M. Role of point-of-care ultrasound study in early disposition of patients with undifferentiated acute dyspnea in emergency department: A multi-center prospective study. J. Ultrasound 2022, 25, 443–449.
- Baid, H.; Vempalli, N.; Kumar, S.; Arora, P.; Walia, R.; Chauhan, U.; Shukla, K.; Verma, A.; Chawang, H.; Agarwal, D. Point of care ultrasound as initial diagnostic tool in acute dyspnea patients in the emergency department of a tertiary care center: Diagnostic accuracy study. Int. J. Emerg. Med. 2022, 15, 27.
- Tuinman, P.R.; Jonkman, A.H.; Dres, M.; Shi, Z.H.; Goligher, E.C.; Goffi, A.; de Korte, C.; Demoule, A.; Heunks, L. Respiratory muscle ultrasonography: Methodology, basic and advanced principles and clinical applications in ICU and ED patients—A narrative review. Intensive Care Med. 2020, 46, 594–605.
- Kilaru, D.; Panebianco, N.; Baston, C. Diaphragm Ultrasound in Weaning from Mechanical Ventilation. Chest 2021, 159, 1166–1172.
- Haji, K.; Royse, A.; Green, C.; Botha, J.; Canty, D.; Royse, C. Interpreting diaphragmatic movement with bedside imaging, review article. J. Crit. Care 2016, 34, 56–65.
- Chu, S.E.; Lu, J.X.; Chang, S.C.; Hsu, K.H.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.; Ng, C.J.; Seak, C.J. Point-of-care application of diaphragmatic ultrasonography in the emergency department for the prediction of development of respiratory failure in community-acquired pneumonia: A pilot study. Front. Med. 2022, 9, 960847.
- Jia, Y.; Zhang, Q. Research Progress on Diaphragm Ultrasound in Chronic Obstructive Pulmonary Disease: A Narrative Review. Ultrasound Med. Biol. 2022, 48, 587–597.
- Laverdure, F.; Genty, T.; Rezaiguia-Delclaux, S.; Herve, P.; Stephan, F. Ultrasound Assessment of Respiratory Workload with High-Flow Nasal Oxygen Versus Other Noninvasive Methods After Chest Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3042–3047.
- Shrestha, G.S. Point-of-care ultrasonography and liberation from mechanical ventilation. Crit. Care 2017, 21, 54.
