Anaplastic lymphoma kinase (ALK+ NSCLC)+ Non-small cell lung cancer (NSCLC), affecting about 5% of cases, is characterized by a mutation in the ALK gene, leading to poor life expectancy and a high risk of brain metastases. Unmet needs in metastatic NSCLC include the development of treatments that improve survival, reduce toxicity, and effectively address brain metastases. Evaluating FDAood and Drug Administration (FDA)-approved ALK inhibitors in advanced NSCLC highlights their unique effectiveness and safety profiles. Crizotinib exhibits notable benefits regarding PFS and ORRprogression-free survival (PFS) and objective response rate (ORR); however, multiple studies consistently position alectinib as the superior option. Alectinib distinguishes itself with extended PFS, increased CNScentral nervous system (CNS) activity, and excellent patient-reported outcomes.
- NSCLC
- ALK inhibitors
- lung cancer
- targeted therapy
1. Introduction
2. The ALK Structural Biology
2.1. ALK Extracellular Side
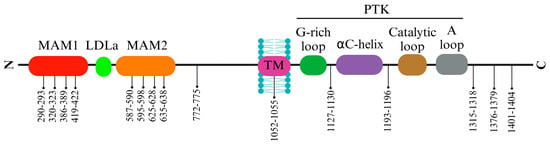
The ALK extracellular domain (ECD) consists of distinct segments believed to serve specific roles such as binding ligands, interacting with potential co-receptors and secreted regulatory proteins, and facilitating dimerization. These functions could trigger structural changes initiating activation within the intracellular protein tyrosine kinase (PTK) domain. The ECD of ALK stands out among receptor tyrosine kinases (RTKs) due to its distinct glycine-rich section. At the same time, ALK includes an additional low-density lipoprotein receptor class A (LDLa) and two meprin, A5 protein, and receptor protein tyrosine phosphatase mu (MAM) domains (Figure 1). Pleiotrophin (PTN) and midkine (MK) are recognized as triggers for mammalian ALK, playing pivotal roles in neural development, survival, and tumorigenesis [28][17]. These growth factors, binding to heparin, can activate various receptors, including receptor protein tyrosine phosphatase-β (RPTPβ), RPTPζ, N-syndecan, low-density lipoprotein receptor-related protein (LRP), and integrins. PTN can specifically engage RPTPβ and RPTPζ phosphatases to initiate ALK signaling.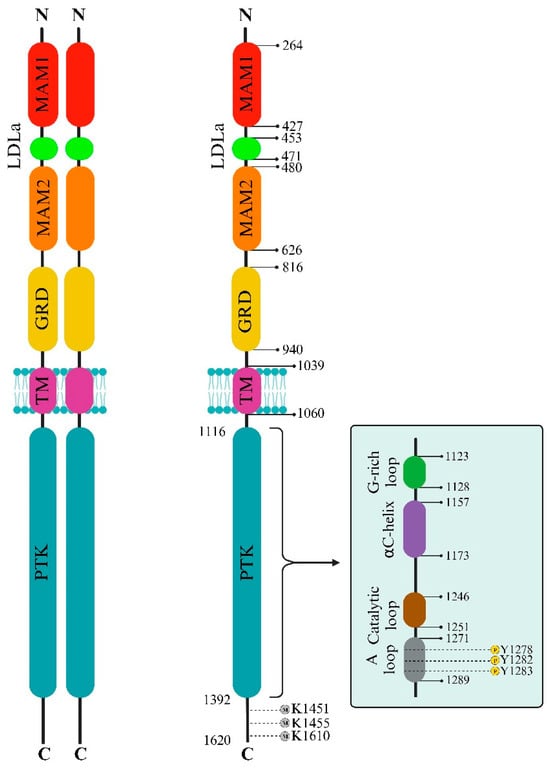
2.2. ALK Intracellular Side
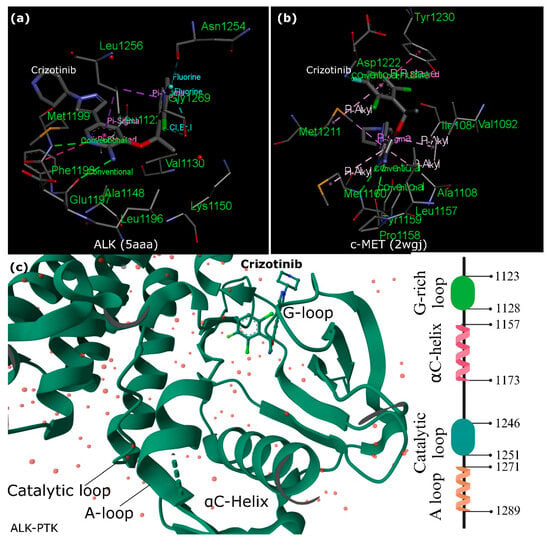
2.3. Crizotinib and ALK Inhibition versus c-MET
3. ALK Cleavage and Modifications
4. ALK Signaling and TKI Resistance
The majority of cases in NSCLC include a subset of two to seven percent of patients exhibiting gene rearrangements of the ALK gene or chromosomal fusions of ALK with echinoderm microtubule-associated protein-like 4 (EML4) [55,56][39][40]. Using ALK-TKIs has significantly improved the outcomes for NSCLC patients with these specific genetic abnormalities. Nevertheless, emerging evidence underscores the clinical challenge of primary or secondary resistance to ALK inhibitors during treatment, necessitating a shift to second- or third-generation ALK-TKIs and the meticulous monitoring of NSCLC patients on ALK-TKIs through repeated molecular testing [57][41]. The latest generation of ALK-TKIs offers benefits for most individuals with EML4-ALK fusions. While several ALK inhibitors, such as crizotinib, alectinib, and ceritinib, have been utilized clinically for ALK+ NSCLC treatment, resistance commonly develops against these inhibitors. The mutated forms of ALK, along with ALK fusion proteins such as NPM-ALK, can activate various signaling pathways that contribute to cell transformation and the maintenance of a cancerous state. This persistent activation triggers the recruitment of several adaptors, initiating multiple signaling pathways. Mutated ALK and ALK chimeras induce mitogenic signaling, predominantly through the RAS/mitogen-activated protein (MAP) kinase pathway, facilitated by the direct binding of IRS1, SHC, and SRC to specific tyrosine residues within ALK’s intracytoplasmic segment. The SHP2/growth factor receptor-bound protein 2 (GRB2) complex interaction with p130Cas alters cytoskeletal organization. Activation of the phosphatidylinositol 3 kinase (PI3K) pathway by ALK results in a significant anti-apoptotic signal, mainly mediated by pAKT1/2 and its downstream molecules that inhibit BAD and FOXO3a-mediated transcription, while regulating cell cycle progression. Further, in neuroendocrine prostate cancer (NEPC), a severe form of prostate cancer, a mutation (ALK F1174C) in the ALK gene responded well to alectinib. An experimental model combining ALK F1174C and N-Myc led to aggressive NEPC, mirroring poor outcomes seen in human datasets. This combination also activated the wnt/β-catenin pathway [64][42]; however, as mentioned earlier, ALK cleavage by MMP-9 in neuroblastoma results in β-catenin release from ALK [51][36]. Further investigation revealed that ALK controls MYC’s transcriptional expression and activates c-MYC’s regulation of target genes in NSCLC. Silencing MYC, either through RNAi or small molecules, sensitizes ALK+ cells to crizotinib. These findings illuminate a dual oncogenic mechanism whereby ALK stimulates the MYC signaling axis, suggesting that targeting MYC could potentially prevent or overcome crizotinib resistance [65][43]. In addition, findings suggested that targeting Src signaling could be a promising therapeutic strategy for ALK+ NSCLC cases that have developed resistance to ALK-TKIs. Researchers discovered that Src signaling is a key resistance mechanism to alectinib, and combining ALK and Src inhibitors effectively halted the growth of ALK-TKI-resistant cells. Further, blocking Src in alectinib-resistant cells effectively countered the activation of phospho-receptor tyrosine kinases and downstream PI3K/AKT signaling. This combined inhibition of ALK and Src also displayed effectiveness against other ALK+ NSCLC cell lines resistant to ceritinib or lorlatinib [66][44]. On the other hand, in NSCLC, where ALK genes are rearranged, resistance to ALK-TKIs remains a challenge despite their success. Research into resistance mechanisms uncovered a new adaptive resistance mechanism linked to JNK/c-Jun signaling. This pathway contributes to the survival of cells tolerant to alectinib and brigatinib. Blocking JNK/c-Jun improved the effectiveness of ALK-TKI treatment in curbing cell growth and promoting cell death. Combining the inhibition of JNK with ALK-TKIs increased cell death by suppressing Bcl-xL proteins, surpassing the effects observed with ALK-TKI treatment alone. Targeting both JNK signaling and ALK might be a promising method to improve outcomes for ALK-rearranged NSCLC [68][45]. Additionally, treatment advancements for NSCLC with the EML4-ALK fusion gene have been made with ALK-TKIs. In ALK-TKI-resistant cells, the expression of EML4-ALK decreased at the transcriptional level, while the phosphorylation of EGFR, HER2, and HER3 increased compared to parental-sensitive cells. This increase in the activation of HER family proteins coincided with a higher secretion of EGF. Treatment with an EGFR-TKI induced apoptosis in ALK-TKI resistant cells but not in sensitive cells. In the parental cells, the inhibition of extracellular signal-regulated kinase (ERK) and STAT3 phosphorylation by the selective ALK-TKI TAE684 was disrupted when these cells were exposed to exogenous EGF, leading to reduced sensitivity in cell growth to TAE684 [69][46]. However, resistance, notably the G1202R mutation in ALK, limits their effectiveness. In addition, research exploring protein methylation, notably SET and MYND domain containing 2 (SMYD2) methyltransferases, discovered their role in methylating specific lysine residues (1451, 1455, and 1610) in the ALK protein. Lowering SMYD2 levels or using an SMYD2 inhibitor reduced EML4-ALK protein phosphorylation in NSCLC cell lines. Modification of these lysine residues hindered ALK methylation and inhibited downstream AKT phosphorylation, impeding cell growth. Combining SMYD2 and ALK inhibitors demonstrated enhanced efficacy in restraining NSCLC cell growth. Hence, this SMYD2-mediated ALK methylation is suggested as a novel treatment avenue for ALK fusion gene-related tumors [72][47].5. FDA-Approved ALK Inhibitors
5.1. Main Clinical Trials
Studies comparing various ALK inhibitors for advanced NSCLC have highlighted distinct efficacy profiles and safety concerns associated with each medication. Examining the efficacy of crizotinib, alectinib, brigatinib, ceritinib, and lorlatinib in treating ALK+ NSCLC has provided valuable insights into their performance, safety, and unique adverse event profiles [80][48]. Clinical studies [81][49] highlighted crizotinib’s superior performance over chemotherapy, emphasizing extended PFS and higher ORR. Similarly, another research [82][50] reinforced these findings in PROFILE 1029 (NCT01639001), underlining the significant advantages of crizotinib in terms of PFS, ORR, and prompt response time among East Asian patients despite negligible differences in overall survival (OS). Conversely, several studies have consistently drawn attention to alectinib’s advantages compared to crizotinib. The global phase III ALEX study showcased notable enhancements in PFS and OS when comparing alectinib to crizotinib in treatment-naive individuals with ALK + NSCLC. Subsequent phase III trials in Japanese and Asian populations (J-ALEX and ALESIA) affirmed the clinical advantages of alectinib over crizotinib as a first-line therapy. On the other hand, in the ALTA-1L trial (NCT02737501), brigatinib’s effectiveness was evaluated against crizotinib among patients with locally advanced or metastatic ALK+ NSCLC who had not previously received ALK inhibitors [88][51]. Brigatinib demonstrated significant superiority over crizotinib in terms of PFS and ORR. The trial highlighted brigatinib’s substantial increase in response duration compared to crizotinib, with an OS rate of 86% for crizotinib and 85% for brigatinib. Notably, the adverse effect profiles differed between the two treatments, with distinct patterns of side effects observed in each group. In the multicenter, randomized ASCEND-4 trial (NCT01828099) comparing ceritinib to platinum-based chemotherapy, the efficacy and safety of ceritinib in ALK-rearranged nonsquamous NSCLC were assessed [93][52]. Ceritinib demonstrated significantly longer PFS than chemotherapy, with substantial, rapid, and durable responses observed in the ceritinib group. However, adverse events, particularly diarrhea, nausea, and vomiting, were more familiar with ceritinib, including higher-grade events such as elevated liver enzymes. The effectiveness and tolerability of ceritinib were further analyzed in a Japanese subgroup of patients from the ASCEND-5 (NCT01828112) study [21,94][12][53]. The ceritinib group exhibited prolonged PFS compared to chemotherapy, though it came with a higher incidence of suspected drug-related adverse events. In the CROWN clinical trial (NCT03052608), a phase 3, open-label, multicenter, randomized study, researchers investigated the efficacy of lorlatinib as a first-line treatment for advanced ALK+ NSCLC in comparison to crizotinib [96][54]. The trial enrolled 296 patients without previously received systemic therapy for their metastatic disease. Patients were randomized to receive either lorlatinib (100 mg daily) or crizotinib (250 mg twice daily) for 28 days. The study revealed significant advantages in favor of lorlatinib over crizotinib. The proportion of patients alive at 12 months without disease progression was notably higher in the lorlatinib arm compared to the crizotinib arm (78% versus 39%, p < 0.001).5.2. Efficacy and Tolerability Profiles
In a clinical study involving 72 Chinese patients diagnosed with ALK+ NSCLC, crizotinib demonstrated favorable effectiveness and was well-tolerated. Administered orally at 250 mg twice daily, the patients, primarily characterized as young, non/light smokers with adenocarcinoma histology, achieved an ORR of 52.2% and a disease control rate of 64.2%. Common adverse effects included mild visual disturbances, nausea, vomiting, diarrhea, and constipation. The findings suggest that crizotinib is well-tolerated and effective in this patient cohort, highlighting the need for further prospective, multicenter studies with larger sample sizes to validate these results [99][55]. Furthermore, lorlatinib, identified as a potent and highly active ALK inhibitor with favorable outcomes in later-line settings, aligns with clinical trial data. In a real-world evaluation of 38 heavily pretreated patients with ALK + NSCLC, lorlatinib demonstrated notable efficacy and tolerability. The overall response rate was 44%, and the disease control rate was 81%, indicating substantial antitumor activity. Lorlatinib dose adjustments, including reduction (18%), interruption (16%), and discontinuation (3%), were consistent with the trial experience. Median OS from advanced ALK+ diagnosis ranged from 45.0 to 69.9 months, while median PFS from lorlatinib initiation varied from 7.3 to 27.7 months. In conclusion, examining various ALK inhibitors for advanced NSCLC has unveiled their distinct efficacy profiles and safety considerations. While crizotinib showcased notable advantages regarding PFS and ORRs, alectinib consistently emerged as a superior performer across multiple studies. Alectinib’s prolonged PFS heightened CNS activity, and excellent patient-reported outcomes stood out prominently. Despite its effectiveness in extending PFS, brigatinib exhibited more adverse effects than crizotinib. Ceritinib’s ability to improve PFS and reduce the risk of disease progression or mortality underscored its clinical significance. As a first-line treatment, lorlatinib demonstrated promising outcomes, displaying superior PFS and a higher proportion of patients without disease progression at the 12-month than crizotinib. However, it is essential to note that lorlatinib showed varying adverse effect profiles and impacts on different facets of patients’ quality of life. These findings provide clinicians with invaluable insights to tailor therapies according to individual patient needs and tolerability, ultimately advancing the development of personalized treatment strategies for ALK+ NSCLC.6. Advancements in ALK-Targeted Therapy and Future Horizons
6.1. ALK and Immunotherapy
Changes in the structure of the ALK gene significantly contribute to the onset of different human cancers, and therapies aimed at this gene have revolutionized how we treat these tumors driven by this specific oncogene. However, overcoming inherent or acquired resistance remains a significant hurdle. Variations in the ALK gene, such as gene rearrangements or mutations, also influence the immune environment within tumors. Harnessing immunotherapy to target the ALK gene holds promise in clinical settings [10][56]. The association between ALK rearrangement and immune cells is complex and contingent on the specific characteristics of the tumor microenvironment. In ALK + NSCLC, the proportion of tumors expressing PD-L1 was lower compared to KRAS + NSCLC. Furthermore, T cells expressing immune checkpoint proteins, including T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), CTLA4, Lymphocyte activation gene 3 (LAG3), and PD-1, were less prevalent in ALK+ NSCLC than in EGFR/KRAS + NSCLC. Additionally, the levels of CD3, CD8 T cells, and CD20 B cells were lower in ALK+ NSCLC compared to KRAS+ NSCLC, while CD4 helper T cell levels were higher in ALK+ NSCLC than in EGFR/KRAS+ NSCLC. TIM3 repression was higher in ALK+ NSCLC than in KRAS+ NSCLC. Notably, high expression of PD-L1 and CTLA4 was associated with lower OS in advanced-stage ALK-rearranged NSCLC patients treated with ALK-TKIs. These findings suggest an immunosuppressive tumor microenvironment in ALK + NSCLC, emphasizing the need for further exploration and validation of immunotherapy in this patient population through clinical trials [105][57]. Additionally, individuals with EGFR mutations or ALK rearrangements exhibited the lowest proportion of tumors expressing both PD-L1 and CD8 (PD-L1+/CD8+), at 5.0%. In contrast, at 63.5%, the highest proportion was observed in tumors lacking both PD-L1 and CD8 expression (PD-L1-/CD8-). Conversely, those with wild-type EGFR and ALK presented 14.2% of tumors showing PD-L1+/CD8+ and 50.3% with PD-L1-/CD8-. Consequently, patients harboring EGFR mutations or ALK rearrangements demonstrated a diminished PD-L1 and CD8 co-expression level in the tumor microenvironment, potentially contributing to an inadequate response to ICIs. The co-expression of PD-L1 and CD8 in EGFR-mutated or ALK-rearranged lung cancer serves as a biomarker for poor prognosis, correlating with a shorter OS [106][58]. On the other hand, interleukin (IL)-6, IL-8, and IL-10 have been associated with disease progression in NSCLC, specifically in ALK+ patients. The interactions between TLRs and various interleukins underscore their involvement in lung cancer pathogenesis, progression, and potential prognostic value [108][59]. Moreover, serum-soluble IL-2R levels are a reliable marker for disease activity in hairy cell leukemia and adult T-cell leukemia/lymphoma patients. In addition, ALCL patients often display CD30 and CD25 expression in malignant cells. The occurrence of any mutation in ALK results in the promotion of PD-L1 expression. Increasing the expression of immunosuppressive molecules such as PD-L1 may lead to tolerance and immune evasion in patients with tumors and cancers. Tian et al. have shown that upregulation of PD-L1 can be identified as a biomarker for ALK-rearrange NSCLC. In addition, it has been recognized that the TME in the presence of upregulated expression of PD-L1 encompasses an immunosuppressive condition [113,114][60][61]. ICIs have shown significant promise in various cancers [115,116][62][63]. In ALK+ NSCLC, these inhibitors have been explored, mainly due to the upregulation of PD-L1 expression in ALK+ tumors [117,118][64][65]. However, studies on the prognosis of ALK+ patients using ICIs have yielded conflicting results, necessitating further investigation [117][64]. Initial data from randomized studies suggested lower effectiveness of immunotherapies in ALK+ tumors compared to wild-type tumors. In addition, another study studied how ALK fusion proteins regulate PD-L1 expression and immune function in ALK+ NSCLC. Researchers observed a correlation between PD-L1 expression, EGFR mutations, and ALK fusion genes in NSCLC cell lines. Elevating ALK fusion protein levels boosted PD-L1 expression, leading to T-cell apoptosis in co-culture systems. Blocking ALK with TKIs amplified IFN-γ production. Anti-PD-1 antibodies were effective in both crizotinib-sensitive and -resistant NSCLC cells. However, combining ALK-TKIs with anti-PD-1 antibodies did not benefit co-culture systems. ALK-TKIs suppressed tumor growth and indirectly bolstered antitumor immunity by reducing PD-L1 expression.6.2. Molecular Diagnosis of ALK: Insights from Next-Generation Sequencing
Molecular analyses, mainly focusing on genetic rearrangements in genes such as ALK, ROS1, RET, and NTRK [135][66], have become standard practices in patients with advanced NSCLC—immunohistochemistry (IHC) functions as the primary screening method, valued for its ease of implementation and interpretation. Fluorescence in situ hybridization (FISH) confirms rearrangements, especially in cases with ambiguous immunostainings. Although FISH is acknowledged as the most sensitive method for detecting ALK and ROS1 rearrangements, it requires comprehensive guidelines for result interpretation [136][67]. On the other hand, advanced genomic analyses, such as next-generation sequencing (NGS), meticulously scrutinize the genetic composition of NSCLC. The pivotal roles of ALK, ROS1, and RET genes in NSCLC development make their fusion events crucial for targeted therapies. Researchers characterize these fusion events by employing cutting-edge techniques, aiming for a profound understanding of the molecular intricacies driving NSCLC progression. The expanding coverage of genetic testing has led to the discovery of numerous ALK fusion subtypes and partners, with over 90 rare ALK fusion subtypes identified in NSCLC. While common fusions such as EML4-ALK have established clinical data, rare fusions such as striatin (STRN)-ALK and huntingtin interacting protein 1 (HIP1)-ALK lack substantial clinical evidence. ALK-TKIs are clinically applied based on ALK gene positivity, irrespective of the fusion partner [137,138][68][69]. The research utilized target-capture DNA NGS to identify ALK, ROS1, and RET fusions in NSCLC, examining genomic breakpoints as predictors of targeted therapy efficacy. Categorizing canonical and uncommon fusions among 3787 samples based on breakpoint positions, RNA sequencing revealed 12.8% of uncommon fusions as nonproductive. The study stressed unreliable efficacy prediction for uncommon genomic breakpoints, recommending RNA or protein validation [139][70]. In another investigation, a hybridization-based NGS approach on 302 NSCLC tumors identified three non-EML4-ALK fusions and additional fusions through RNA sequencing, emphasizing NGS as promising for ambiguous cases and novel fusion detection [140][71].6.3. ALK and Co-Targeting Approaches
The continuous evolution of ALK inhibitors has significantly improved PFS in NSCLC. Notably, second- and third-generation inhibitors such as brigatinib and lorlatinib exhibit remarkable efficacy in controlling brain metastases. The shift toward personalized medicine, involving genetic panels for diagnosis and tailored targeted therapies, represents a new paradigm. Adopting broad molecular panels as the standard of care will facilitate the detection of resistance mechanisms. This prolonged PFS is anticipated to transform the disease into a manageable, chronic condition. Effective treatment sequencing will be vital for patient survival, and the potential replacement of tissue biopsies with liquid biopsies is on the horizon [156][72]. In addition, clinical trials have shown that ALK inhibitors exhibit excellent efficacy against brain metastases. Consequently, initiating treatment with these specific inhibitors is considered reasonable in asymptomatic patients. Radiotherapy can then be employed during tumor progression or when symptoms arise, ensuring the best possible quality of life for patients [157,158][73][74]. Lineage transformation, recognized as a resistance mechanism to ALK-TKIs, occurs at a low frequency, less than 5%, and is primarily attributed to changes in transcriptional patterns rather than acquiring new genomic mutations in the cells [160][75]. In cases of resistance to second-generation ALK-TKIs, treatment strategies should be personalized according to the identified resistance mechanisms. Lorlatinib is the preferred option for patients with ALK mutations resistant to these TKIs, providing comprehensive coverage, including mutations such as G1202R and L1196M. For situations without specific resistance mutations, alternative options such as atezolizumab, bevacizumab, and platinum-based chemotherapy may be explored [161][76]. In cases of oligo-progression, the approach may involve maintaining the existing systemic treatment despite progression, accompanied by adding local therapies to address advancing lesions. Strategies to counteract on-target resistance mechanisms in ALK-TKI resistance include developing 4th generation TKIs (such as TPX-0131 and NVL-655) and proteolysis-targeting chimeras (PROTACs). In off-target (ALK-independent) resistance cases, potential options include combination therapies targeting ALK along with other downstream or parallel pathways, novel antibody-drug conjugates, or combining ALK inhibitors with chemotherapy and immunotherapy [162][77]. Crizotinib exhibits notable efficacy in ALK+ lung cancers, but variable responses and acquired resistance pose challenges. Clinical observations of an exceptional response to an insulin-like growth factor 1 receptor (IGF-1R)-specific antibody in an ALK+ patient led to the identification of therapeutic synergism between ALK and IGF-1R inhibitors. ALK fusion proteins bind to insulin receptor substrate 1 (IRS-1), and inhibiting IRS-1 enhances ALK inhibitors’ antitumor effects. In models of ALK-TKI resistance, activation of the IGF-1R pathway is observed, and combined ALK and IGF-1R inhibition improves therapeutic efficacy. Biopsy samples from patients progressing on crizotinib monotherapy show increased levels of IGF-1R and IRS-1, suggesting a role for the IGF-1R-IRS-1 pathway in both ALK-TKI-sensitive and ALK-TKI-resistant states, supporting further clinical development of dual ALK and IGF-1R inhibitors [163][78]. An extraordinary responder in a trial employing erlotinib and IGF-1R antibody unveiled a synergistic impact between ALK and IGF-1R inhibitors. Despite the initial unresponsiveness of the patient’s tumor to erlotinib alone, a remarkable 17-month response emerged with the combination. As subsequent molecular profiling identified an ALK rearrangement, the study proposed the IGF-1R–IRS-1 signaling axis as a potential therapeutic focus in ALK+ lung cancer, providing insights for upcoming clinical trials [164][79]. Furthermore, co-targeting primary anticancer targets and corresponding drug escape pathways might enhance anticancer therapeutics. The clinical status and targets of 23 approved and 136 clinical trial multi-target anticancer drugs, focusing on co-targeting ALK, EGFR, HER2, Abl, VEGFR2, mTOR, PI3K, MEK, KIT, and DNA topoisomerase, demonstrated that the majority of approved (73.9%) and phase 3 (75.0%) drugs, as well as a significant portion of phase 2 (62.8%) and phase 1 (53.6%) drugs, co-targeted cancer drug escape pathways, suggesting a potential clinical advantage in co-targeting anticancer targets and drug escape pathways, encouraging further exploration of this strategy [166][80].6.4. Other ALK-Innovative Approaches
In ALK+ cancer models, DNA vaccines directed against the ALK gene exhibited notable effectiveness [174][81]. These vaccines prompted specific immune reactions against ALK, fostering CD8+ T cell-mediated cytotoxicity and provoking IFN-γ responses. When combined with chemotherapy, ALK-DNA vaccination significantly extended the survival of mice afflicted with ALK+ lymphomas. In the context of ALK+ NSCLC models, the ALK-DNA vaccine triggered robust immune responses, curtailing tumor growth and elongating survival. However, lung tumors with ALK rearrangements establish an immunosuppressive setting, diminishing the efficacy of the ALK vaccine by upregulating PD-L1 expression. ALK vaccine pairing with ALK-TKIs notably delayed tumor relapse post-TKI treatment. Further research explored the treatment of ALK-rearranged NSCLC with ALK-TKI and ICIs. The findings revealed that ICIs were ineffective in prompting the rejection of ALK+ lung tumors. However, a vaccination with a single ALK peptide successfully reinstated the activation of ALK-specific CD8+ T cells. When coupled with ALK-TKIs, this vaccination eradicated lung tumors and impeded metastatic spread to the brain. The research additionally pinpointed human ALK peptides suitable for vaccination, demonstrating their immunogenicity in mice and their recognition by CD8+ T cells in individuals with NSCLC. This breakthrough implies the potential development of a clinical vaccine for treating ALK+ NSCLC [176][82]. Additionally, alternative ALK vaccines utilizing peptides or lipid vesicles encapsulating ALK antigens showcased potential in restraining tumor advancement in preclinical models. Further, the application of anti-EGF-VacAbs targeting EGF in ALK+ NSCLC cell lines amplified the effectiveness of ALK-TKIs, impeding the emergence of resistance and intercepting downstream oncogenic pathways [177][83]. On the other hand, scientists developed a highly sensitive NanoBiT LATS bioluminescent biosensor (BS) to track LATS kinase activity in the Hippo signaling pathway in lab settings and living organisms. This new biosensor showed greater sensitivity and stability than previous versions, even when expressed at significantly lower levels. Using this advanced biosensor, they could monitor LATS activity in live cells at physiologically relevant levels and simplify kinase activity analysis in vitro. Alternatively, researchers recently investigated the role of the Nuclear Interaction Partner of ALK (NIPA) in a specific type of lymphoma induced by the NPM-ALK gene. Previous studies highlighted NIPA’s significance in cell division control and bone marrow failure but had yet to explore its involvement in NPM-ALK-driven lymphomas [179][84]. Researchers demonstrated that NIPA interacts with NPM-ALK, and its absence or downregulation led to significant impairment in the growth and transformation of cells associated with this lymphoma in lab tests. Furthermore, T-LAK cell-oriented protein kinase (TOPK), recognized as a potential therapeutic target in cancer, has been scrutinized in ALK+ NSCLC. The study identified ALK as an upstream kinase of TOPK, phosphorylating it specifically at Y74. This phosphorylation notably promotes tumor growth in ALK+ lung cancer cells, a finding supported by a phosphoproteomic analysis delineating downstream pathway involvement [181][85]. Comparatively, TOPK emerges as a superior target for cancer therapy compared to other direct downstream molecules of ALK, including Smad4, STAT3, PI3K, and PLC-γ. Clinical studies have consistently associated TOPK with a marker of poor prognosis in various cancers and an independent predictor for OS [182,183,184,185][86][87][88][89]. Encouragingly, inhibitors such as HI-032 and SKLB-C05, which target TOPK, have demonstrated promising potential. Moreover, combining TOPK inhibition with alectinib, an ALK inhibitor, has shown remarkable synergy in impeding cell proliferation and promoting apoptosis. This combined approach proposes a promising strategy to counter drug resistance in ALK+ NSCLC [181][85]. These research findings advance our understanding of ALK’s oncogenic signaling network and suggest the potential efficacy of co-inhibition of ALK and TOPK as a novel therapeutic strategy to treat ALK+ NSCLC and potentially delay the onset of drug resistance.References
- Panagiotidis, E. The Role of Positron Computed Tomography (PET/CT) in Lung Cancer Staging. Hell. J. Nucl. Med. 2023, 26, 22–29.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Hallberg, B.; Palmer, R.H. Mechanistic Insight into ALK Receptor Tyrosine Kinase in Human Cancer Biology. Nat. Rev. Cancer 2013, 13, 685–700.
- Pearson, J.D.; Lee, J.K.H.; Bacani, J.T.C.; Lai, R.; Ingham, R.J. NPM-ALK: The Prototypic Member of a Family of Oncogenic Fusion Tyrosine Kinases. J. Signal Transduct. 2012, 2012, 123253.
- Reshetnyak, A.V.; Rossi, P.; Myasnikov, A.G.; Sowaileh, M.; Mohanty, J.; Nourse, A.; Miller, D.J.; Lax, I.; Schlessinger, J.; Kalodimos, C.G. Mechanism for the Activation of the Anaplastic Lymphoma Kinase Receptor. Nature 2021, 600, 153–157.
- Huang, H. Anaplastic Lymphoma Kinase (ALK) Receptor Tyrosine Kinase: A Catalytic Receptor with Many Faces. Int. J. Mol. Sci. 2018, 19, 3448.
- Andraos, E.; Dignac, J.; Meggetto, F. NPM-ALK: A Driver of Lymphoma Pathogenesis and a Therapeutic Target. Cancers 2021, 13, 144.
- Silva, A.P.S.; Coelho, P.V.; Anazetti, M.; Simioni, P.U. Targeted Therapies for the Treatment of Non-Small-Cell Lung Cancer: Monoclonal Antibodies and Biological Inhibitors. Hum. Vaccines Immunother. 2017, 13, 843–853.
- Jahanzeb, M.; Lin, H.M.; Pan, X.; Yin, Y.; Baumann, P.; Langer, C.J. Immunotherapy Treatment Patterns and Outcomes Among ALK-Positive Patients with Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 49–57.
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328.
- Tabbò, F.; Passiglia, F.; Novello, S. Upfront Management of ALK-Rearranged Metastatic Non-Small Cell Lung Cancer: One Inhibitor Fits All? Curr. Oncol. Rep. 2021, 23, 10.
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus Chemotherapy in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer Previously given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet. Oncol. 2017, 18, 874–886.
- Gadgeel, S.M.; Gandhi, L.; Riely, G.J.; Chiappori, A.A.; West, H.L.; Azada, M.C.; Morcos, P.N.; Lee, R.-M.; Garcia, L.; Yu, L.; et al. Safety and Activity of Alectinib against Systemic Disease and Brain Metastases in Patients with Crizotinib-Resistant ALK-Rearranged Non-Small-Cell Lung Cancer (AF-002JG): Results from the Dose-Finding Portion of a Phase 1/2 Study. Lancet. Oncol. 2014, 15, 1119–1128.
- Ou, S.-H.I.; Ahn, J.S.; De Petris, L.; Govindan, R.; Yang, J.C.-H.; Hughes, B.; Lena, H.; Moro-Sibilot, D.; Bearz, A.; Ramirez, S.V.; et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J. Clin. Oncol. 2016, 34, 661–668.
- Novello, S.; Mazières, J.; Oh, I.-J.; deCastro, J.; Migliorino, M.R.; Helland, Å.; Dziadziuszko, R.; Griesinger, F.; Kotb, A.; Zeaiter, A.; et al. Alectinib versus Chemotherapy in Crizotinib-Pretreated Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small-Cell Lung Cancer: Results from the Phase III ALUR Study. Ann. Oncol. 2018, 29, 1409–1416.
- Kim, D.-W.; Tiseo, M.; Ahn, M.-J.; Reckamp, K.L.; Hansen, K.H.; Kim, S.-W.; Huber, R.M.; West, H.L.; Groen, H.J.M.; Hochmair, M.J.; et al. Brigatinib in Patients with Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2017, 35, 2490–2498.
- Palmer, R.H.; Vernersson, E.; Grabbe, C.; Hallberg, B. Anaplastic Lymphoma Kinase: Signalling in Development and Disease. Biochem. J. 2009, 420, 345–361.
- Li, T.; Stayrook, S.E.; Tsutsui, Y.; Zhang, J.; Wang, Y.; Li, H.; Proffitt, A.; Krimmer, S.G.; Ahmed, M.; Belliveau, O.; et al. Structural Basis for Ligand Reception by Anaplastic Lymphoma Kinase. Nature 2021, 600, 148–152.
- Guan, J.; Umapathy, G.; Yamazaki, Y.; Wolfstetter, G.; Mendoza, P.; Pfeifer, K.; Mohammed, A.; Hugosson, F.; Zhang, H.; Hsu, A.W.; et al. FAM150A and FAM150B Are Activating Ligands for Anaplastic Lymphoma Kinase. Elife 2015, 4, e09811.
- Reshetnyak, A.V.; Murray, P.B.; Shi, X.; Mo, E.S.; Mohanty, J.; Tome, F.; Bai, H.; Gunel, M.; Lax, I.; Schlessinger, J. Augmentor α and β (FAM150) Are Ligands of the Receptor Tyrosine Kinases ALK and LTK: Hierarchy and Specificity of Ligand-Receptor Interactions. Proc. Natl. Acad. Sci. USA 2015, 112, 15862–15867.
- Kornev, A.P.; Taylor, S.S. Dynamics-Driven Allostery in Protein Kinases. Trends Biochem. Sci. 2015, 40, 628–647.
- Donella-Deana, A.; Marin, O.; Cesaro, L.; Gunby, R.H.; Ferrarese, A.; Coluccia, A.M.L.; Tartari, C.J.; Mologni, L.; Scapozza, L.; Gambacorti-Passerini, C.; et al. Unique Substrate Specificity of Anaplastic Lymphoma Kinase (ALK): Development of Phosphoacceptor Peptides for the Assay of ALK Activity. Biochemistry 2005, 44, 8533–8542.
- Tartari, C.J.; Gunby, R.H.; Coluccia, A.M.L.; Sottocornola, R.; Cimbro, B.; Scapozza, L.; Donella-Deana, A.; Pinna, L.A.; Gambacorti-Passerini, C. Characterization of Some Molecular Mechanisms Governing Autoactivation of the Catalytic Domain of the Anaplastic Lymphoma Kinase. J. Biol. Chem. 2008, 283, 3743–3750.
- Wang, P.; Wu, F.; Zhang, J.; McMullen, T.; Young, L.C.; Ingham, R.J.; Li, L.; Lai, R. Serine Phosphorylation of NPM-ALK, Which Is Dependent on the Auto-Activation of the Kinase Activation Loop, Contributes to Its Oncogenic Potential. Carcinogenesis 2011, 32, 146–153.
- Hallberg, B.; Palmer, R.H. The Role of the ALK Receptor in Cancer Biology. Ann. Oncol. 2016, 27 (Suppl. 3), iii4–iii15.
- Shaw, A.T.; Friboulet, L.; Leshchiner, I.; Gainor, J.F.; Bergqvist, S.; Brooun, A.; Burke, B.J.; Deng, Y.-L.; Liu, W.; Dardaei, L.; et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N. Engl. J. Med. 2016, 374, 54–61.
- Cui, J.J.; Tran-Dubé, M.; Shen, H.; Nambu, M.; Kung, P.-P.; Pairish, M.; Jia, L.; Meng, J.; Funk, L.; Botrous, I.; et al. Structure Based Drug Design of Crizotinib (PF-02341066), a Potent and Selective Dual Inhibitor of Mesenchymal-Epithelial Transition Factor (c-MET) Kinase and Anaplastic Lymphoma Kinase (ALK). J. Med. Chem. 2011, 54, 6342–6363.
- Kazandjian, D.; Blumenthal, G.M.; Chen, H.-Y.; He, K.; Patel, M.; Justice, R.; Keegan, P.; Pazdur, R. FDA Approval Summary: Crizotinib for the Treatment of Metastatic Non-Small Cell Lung Cancer with Anaplastic Lymphoma Kinase Rearrangements. Oncologist 2014, 19, e5–e11.
- Huang, X. The Potential Role of HGF-MET Signaling and Autophagy in the War of Alectinib versus Crizotinib against ALK-Positive NSCLC. J. Exp. Clin. Cancer Res. 2018, 37, 33.
- Morris, S.W.; Naeve, C.; Mathew, P.; James, P.L.; Kirstein, M.N.; Cui, X.; Witte, D.P. ALK, the Chromosome 2 Gene Locus Altered by the t(2;5) in Non-Hodgkin’s Lymphoma, Encodes a Novel Neural Receptor Tyrosine Kinase That Is Highly Related to Leukocyte Tyrosine Kinase (LTK). Oncogene 1997, 14, 2175–2188.
- Degoutin, J.; Brunet-de Carvalho, N.; Cifuentes-Diaz, C.; Vigny, M. ALK (Anaplastic Lymphoma Kinase) Expression in DRG Neurons and Its Involvement in Neuron-Schwann Cells Interaction. Eur. J. Neurosci. 2009, 29, 275–286.
- Mazot, P.; Cazes, A.; Boutterin, M.C.; Figueiredo, A.; Raynal, V.; Combaret, V.; Hallberg, B.; Palmer, R.H.; Delattre, O.; Janoueix-Lerosey, I.; et al. The Constitutive Activity of the ALK Mutated at Positions F1174 or R1275 Impairs Receptor Trafficking. Oncogene 2011, 30, 2017–2025.
- Pischedda, F.; Ghirelli, A.; Tripathi, V.; Piccoli, G. Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics 2023, 15, 2307.
- Venkannagari, H.; Kasper, J.M.; Misra, A.; Rush, S.A.; Fan, S.; Lee, H.; Sun, H.; Seshadrinathan, S.; Machius, M.; Hommel, J.D.; et al. Highly Conserved Molecular Features in IgLONs Contrast Their Distinct Structural and Biological Outcomes. J. Mol. Biol. 2020, 432, 5287–5303.
- Moog-Lutz, C.; Degoutin, J.; Gouzi, J.Y.; Frobert, Y.; Brunet-de Carvalho, N.; Bureau, J.; Créminon, C.; Vigny, M. Activation and Inhibition of Anaplastic Lymphoma Kinase Receptor Tyrosine Kinase by Monoclonal Antibodies and Absence of Agonist Activity of Pleiotrophin. J. Biol. Chem. 2005, 280, 26039–26048.
- Huang, H.; Gont, A.; Kee, L.; Dries, R.; Pfeifer, K.; Sharma, B.; Debruyne, D.N.; Harlow, M.; Sengupta, S.; Guan, J.; et al. Extracellular Domain Shedding of the ALK Receptor Mediates Neuroblastoma Cell Migration. Cell Rep. 2021, 36, 109363.
- DelGrosso, F.; DeMariano, M.; Passoni, L.; Luksch, R.; Tonini, G.P.; Longo, L. Inhibition of N-Linked Glycosylation Impairs ALK Phosphorylation and Disrupts pro-Survival Signaling in Neuroblastoma Cell Lines. BMC Cancer 2011, 11, 525.
- Contessa, J.N.; Bhojani, M.S.; Freeze, H.H.; Rehemtulla, A.; Lawrence, T.S. Inhibition of N-Linked Glycosylation Disrupts Receptor Tyrosine Kinase Signaling in Tumor Cells. Cancer Res. 2008, 68, 3803–3809.
- Spitaleri, G.; Trillo Aliaga, P.; Attili, I.; Del Signore, E.; Corvaja, C.; Corti, C.; Crimini, E.; Passaro, A.; de Marinis, F. Sustained Improvement in the Management of Patients with Non-Small-Cell Lung Cancer (NSCLC) Harboring ALK Translocation: Where Are We Running? Curr. Oncol. 2023, 30, 5072–5092.
- Lei, Y.; Lei, Y.; Shi, X.; Wang, J. EML4-ALK Fusion Gene in Non-Small Cell Lung Cancer. Oncol. Lett. 2022, 24, 277.
- Smolle, E.; Taucher, V.; Lindenmann, J.; Jost, P.J.; Pichler, M. Current Knowledge about Mechanisms of Drug Resistance against ALK Inhibitors in Non-Small Cell Lung Cancer. Cancers 2021, 13, 699.
- Unno, K.; Chalmers, Z.R.; Pamarthy, S.; Vatapalli, R.; Rodriguez, Y.; Lysy, B.; Mok, H.; Sagar, V.; Han, H.; Yoo, Y.A.; et al. Activated ALK Cooperates with N-Myc via Wnt/β-Catenin Signaling to Induce Neuroendocrine Prostate Cancer. Cancer Res. 2021, 81, 2157–2170.
- Pilling, A.B.; Kim, J.; Estrada-Bernal, A.; Zhou, Q.; Le, A.T.; Singleton, K.R.; Heasley, L.E.; Tan, A.C.; DeGregori, J.; Doebele, R.C. ALK Is a Critical Regulator of the MYC-Signaling Axis in ALK Positive Lung Cancer. Oncotarget 2018, 9, 8823–8835.
- Yoshida, R.; Sasaki, T.; Minami, Y.; Hibino, Y.; Okumura, S.; Sado, M.; Miyokawa, N.; Hayashi, S.; Kitada, M.; Ohsaki, Y. Activation of Src Signaling Mediates Acquired Resistance to ALK Inhibition in Lung Cancer. Int. J. Oncol. 2017, 51, 1533–1540.
- Tanimura, K.; Yamada, T.; Horinaka, M.; Katayama, Y.; Fukui, S.; Morimoto, K.; Nakano, T.; Tokuda, S.; Morimoto, Y.; Iwasaku, M.; et al. Inhibition of C-Jun N-Terminal Kinase Signaling Increased Apoptosis and Prevented the Emergence of ALK-TKI-Tolerant Cells in ALK-Rearranged Non-Small Cell Lung Cancer. Cancer Lett. 2021, 522, 119–128.
- Tanizaki, J.; Okamoto, I.; Okabe, T.; Sakai, K.; Tanaka, K.; Hayashi, H.; Kaneda, H.; Takezawa, K.; Kuwata, K.; Yamaguchi, H.; et al. Activation of HER Family Signaling as a Mechanism of Acquired Resistance to ALK Inhibitors in EML4-ALK–Positive Non–Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 6219–6226.
- Wang, R.; Deng, X.; Yoshioka, Y.; Vougiouklakis, T.; Park, J.-H.; Suzuki, T.; Dohmae, N.; Ueda, K.; Hamamoto, R.; Nakamura, Y. Effects of SMYD2-Mediated EML4-ALK Methylation on the Signaling Pathway and Growth in Non-Small-Cell Lung Cancer Cells. Cancer Sci. 2017, 108, 1203–1209.
- Chazan, G.; Solomon, B.J. Optimal First-Line Treatment for Metastatic ALK+ Non-Small Cell Lung Cancer—A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 369–378.
- Li, J.; Knoll, S.; Bocharova, I.; Tang, W.; Signorovitch, J. Comparative Efficacy of First-Line Ceritinib and Crizotinib in Advanced or Metastatic Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: An Adjusted Indirect Comparison with External Controls. Curr. Med. Res. Opin. 2019, 35, 105–111.
- Wu, Y.-L.; Lu, S.; Lu, Y.; Zhou, J.; Shi, Y.-K.; Sriuranpong, V.; Ho, J.C.M.; Ong, C.K.; Tsai, C.-M.; Chung, C.-H.; et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1539–1548.
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039.
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-Line Ceritinib versus Platinum-Based Chemotherapy in Advanced ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-4): A Randomised, Open-Label, Phase 3 Study. Lancet 2017, 389, 917–929.
- Kiura, K.; Imamura, F.; Kagamu, H.; Matsumoto, S.; Hida, T.; Nakagawa, K.; Satouchi, M.; Okamoto, I.; Takenoyama, M.; Fujisaka, Y.; et al. Phase 3 Study of Ceritinib vs Chemotherapy in ALK-Rearranged NSCLC Patients Previously Treated with Chemotherapy and Crizotinib (ASCEND-5): Japanese Subset. Jpn. J. Clin. Oncol. 2018, 48, 367–375.
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029.
- Cui, S.; Zhao, Y.; Gu, A.; Ge, X.; Song, Y.; Zhang, W.; Lou, Y.; Dong, L.; Han, B.; Jiang, L. Efficacy and Tolerability of Crizotinib in the Treatment of ALK-Positive, Advanced Non-Small Cell Lung Cancer in Chinese Patients. Med. Oncol. 2015, 32, 626.
- Guo, Y.; Guo, H.; Zhang, Y.; Cui, J. Anaplastic Lymphoma Kinase-Special Immunity and Immunotherapy. Front. Immunol. 2022, 13, 908894.
- Zhang, B.; Zeng, J.; Zhang, H.; Zhu, S.; Wang, H.; He, J.; Yang, L.; Zhou, N.; Zu, L.; Xu, X.; et al. Characteristics of the Immune Microenvironment and Their Clinical Significance in Non-Small Cell Lung Cancer Patients with ALK-Rearranged Mutation. Front. Immunol. 2022, 13, 974581.
- Liu, S.-Y.; Dong, Z.-Y.; Wu, S.-P.; Xie, Z.; Yan, L.-X.; Li, Y.-F.; Yan, H.-H.; Su, J.; Yang, J.-J.; Zhou, Q.; et al. Clinical Relevance of PD-L1 Expression and CD8+ T Cells Infiltration in Patients with EGFR-Mutated and ALK-Rearranged Lung Cancer. Lung Cancer 2018, 125, 86–92.
- Mukherjee, S.; Patra, R.; Behzadi, P.; Masotti, A.; Paolini, A.; Sarshar, M. Toll-like Receptor-Guided Therapeutic Intervention of Human Cancers: Molecular and Immunological Perspectives. Front. Immunol. 2023, 14, 1244345.
- Tian, X.; Li, Y.; Huang, Q.; Zeng, H.; Wei, Q.; Tian, P. High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer. Biomolecules 2023, 13, 991.
- Hong, S.; Chen, N.; Fang, W.; Zhan, J.; Liu, Q.; Kang, S.; He, X.; Liu, L.; Zhou, T.; Huang, J.; et al. Upregulation of PD-L1 by EML4-ALK Fusion Protein Mediates the Immune Escape in ALK Positive NSCLC: Implication for Optional Anti-PD-1/PD-L1 Immune Therapy for ALK-TKIs Sensitive and Resistant NSCLC Patients. Oncoimmunology 2016, 5, e1094598.
- Jabbarzadeh Kaboli, P.; Shabani, S.; Sharma, S.; Partovi Nasr, M.; Yamaguchi, H.; Hung, M.-C. Shedding Light on Triple-Negative Breast Cancer with Trop2-Targeted Antibody-Drug Conjugates. Am. J. Cancer Res. 2022, 12, 1671–1685.
- Zhao, Q.; Guo, J.; Zhao, Y.; Shen, J.; Kaboli, P.J.; Xiang, S.; Du, F.; Wu, X.; Li, M.; Wan, L.; et al. Comprehensive Assessment of PD-L1 and PD-L2 Dysregulation in Gastrointestinal Cancers. Epigenomics 2020, 12, 2155–2171.
- Riudavets, M.; Auclin, E.; Mosteiro, M.; Dempsey, N.; Majem, M.; Lobefaro, R.; López-Castro, R.; Bosch-Barrera, J.; Pilotto, S.; Escalera, E.; et al. Durvalumab Consolidation in Patients with Unresectable Stage III Non-Small Cell Lung Cancer with Driver Genomic Alterations. Eur. J. Cancer 2022, 167, 142–148.
- Ota, K.; Azuma, K.; Kawahara, A.; Hattori, S.; Iwama, E.; Tanizaki, J.; Harada, T.; Matsumoto, K.; Takayama, K.; Takamori, S.; et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 4014–4021.
- Marinelli, D.; Siringo, M.; Metro, G.; Ricciuti, B.; Gelibter, A.J. Non-Small-Cell Lung Cancer: How to Manage ALK-, ROS1- and NTRK-Rearranged Disease. Drugs Context 2022, 11.
- Conde, E.; Rojo, F.; Gómez, J.; Enguita, A.B.; Abdulkader, I.; González, A.; Lozano, D.; Mancheño, N.; Salas, C.; Salido, M.; et al. Molecular Diagnosis in Non-Small-Cell Lung Cancer: Expert Opinion on ALK and ROS1 Testing. J. Clin. Pathol. 2022, 75, 145–153.
- Xiang, Y.; Zhang, S.; Fang, X.; Jiang, Y.; Fang, T.; Liu, J.; Lu, K. Therapeutic Advances of Rare ALK Fusions in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 7816–7831.
- Li, M.; An, Z.; Tang, Q.; Ma, Y.; Yan, J.; Chen, S.; Wang, Y. Mixed Responses to First-Line Alectinib in Non-Small Cell Lung Cancer Patients with Rare ALK Gene Fusions: A Case Series and Literature Review. J. Cell. Mol. Med. 2021, 25, 9476–9481.
- Li, W.; Guo, L.; Liu, Y.; Dong, L.; Yang, L.; Chen, L.; Liu, K.; Shao, Y.; Ying, J. Potential Unreliability of Uncommon ALK, ROS1, and RET Genomic Breakpoints in Predicting the Efficacy of Targeted Therapy in NSCLC. J. Thorac. Oncol. 2021, 16, 404–418.
- Wang, B.; Chen, R.; Wang, C.; Guo, J.; Yuan, M.; Chen, H.; Xia, X.; Zhong, D. Identification of Novel ALK Fusions Using DNA/RNA Sequencing in Immunohistochemistry/RT-PCR Discordant NSCLC Patients. Hum. Pathol. 2021, 114, 90–98.
- Zia, V.; Lengyel, C.G.; Tajima, C.C.; de Mello, R.A. Advancements of ALK Inhibition of Non-Small Cell Lung Cancer: A Literature Review. Transl. Lung Cancer Res. 2023, 12, 1563–1574.
- Ceddia, S.; Codacci-Pisanelli, G. Treatment of Brain Metastases in ALK-Positive Non-Small Cell Lung Cancer. Crit. Rev. Oncol. 2021, 165, 103400.
- Wrona, A.; Dziadziuszko, R.; Jassem, J. Combining Radiotherapy with Targeted Therapies in Non-Small Cell Lung Cancer: Focus on Anti-EGFR, Anti-ALK and Anti-Angiogenic Agents. Transl. Lung Cancer Res. 2021, 10, 2032–2047.
- Meador, C.B.; Piotrowska, Z. Biology and Impact of Lineage Plasticity in ALK-Positive NSCLC: A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 837–856.
- Fukuda, A.; Yoshida, T. Treatment of Advanced ALK-Rearranged NSCLC Following Second-Generation ALK-TKI Failure. Expert Rev. Anticancer Ther. 2023, 23, 1157–1167.
- Desai, A.; Lovly, C.M. Strategies to Overcome Resistance to ALK Inhibitors in Non-Small Cell Lung Cancer: A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 615–628.
- Lovly, C.M.; McDonald, N.T.; Chen, H.; Ortiz-Cuaran, S.; Heukamp, L.C.; Yan, Y.; Florin, A.; Ozretić, L.; Lim, D.; Wang, L.; et al. Rationale for Co-Targeting IGF-1R and ALK in ALK Fusion-Positive Lung Cancer. Nat. Med. 2014, 20, 1027–1034.
- Hutchinson, L. Lung Cancer: Combating Resistance through IGF-1R and ALK Co-Targeting. Nat. Rev. Clin. Oncol. 2014, 11, 622.
- Tao, L.; Zhu, F.; Xu, F.; Chen, Z.; Jiang, Y.Y.; Chen, Y.Z. Co-Targeting Cancer Drug Escape Pathways Confers Clinical Advantage for Multi-Target Anticancer Drugs. Pharmacol. Res. 2015, 102, 123–131.
- Chiarle, R.; Martinengo, C.; Mastini, C.; Ambrogio, C.; D’Escamard, V.; Forni, G.; Inghirami, G. The Anaplastic Lymphoma Kinase Is an Effective Oncoantigen for Lymphoma Vaccination. Nat. Med. 2008, 14, 676–680.
- Mota, I.; Patrucco, E.; Mastini, C.; Mahadevan, N.R.; Thai, T.C.; Bergaggio, E.; Cheong, T.-C.; Leonardi, G.; Karaca-Atabay, E.; Campisi, M.; et al. ALK Peptide Vaccination Restores the Immunogenicity of ALK-Rearranged Non-Small Cell Lung Cancer. Nat. Cancer 2023, 4, 1016–1035.
- Codony-Servat, J.; García-Roman, S.; Molina-Vila, M.Á.; Bertran-Alamillo, J.; Viteri, S.; d’Hondt, E.; Rosell, R. Anti-Epidermal Growth Factor Vaccine Antibodies Increase the Antitumor Activity of Kinase Inhibitors in ALK and RET Rearranged Lung Cancer Cells. Transl. Oncol. 2021, 14, 100887.
- Kreutmair, S.; Erlacher, M.; Andrieux, G.; Istvanffy, R.; Mueller-Rudorf, A.; Zwick, M.; Rückert, T.; Pantic, M.; Poggio, T.; Shoumariyeh, K.; et al. Loss of the Fanconi Anemia-Associated Protein NIPA Causes Bone Marrow Failure. J. Clin. Investig. 2020, 130, 2827–2844.
- Xiao, J.; Zhang, L.; Yi, H.; Zou, L.; Mo, J.; Xue, F.; Zheng, J.; Huang, Y.; Lu, H.; Wu, H.; et al. Inhibiting ALK-TOPK Signaling Pathway Promotes Cell Apoptosis of ALK-Positive NSCLC. Cell Death Dis. 2022, 13, 828.
- Zhang, Y.; Yang, X.; Wang, R.; Zhang, X. Prognostic Value of PDZ-Binding Kinase/T-LAK Cell-Originated Protein Kinase (PBK/TOPK) in Patients with Cancer. J. Cancer 2019, 10, 131–137.
- Ikeda, Y.; Park, J.-H.; Miyamoto, T.; Takamatsu, N.; Kato, T.; Iwasa, A.; Okabe, S.; Imai, Y.; Fujiwara, K.; Nakamura, Y.; et al. T-LAK Cell-Originated Protein Kinase (TOPK) as a Prognostic Factor and a Potential Therapeutic Target in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 6110–6117.
- Zlobec, I.; Molinari, F.; Kovac, M.; Bihl, M.P.; Altermatt, H.J.; Diebold, J.; Frick, H.; Germer, M.; Horcic, M.; Montani, M.; et al. Prognostic and Predictive Value of TOPK Stratified by KRAS and BRAF Gene Alterations in Sporadic, Hereditary and Metastatic Colorectal Cancer Patients. Br. J. Cancer 2010, 102, 151–161.
- Ohashi, T.; Komatsu, S.; Ichikawa, D.; Miyamae, M.; Okajima, W.; Imamura, T.; Kiuchi, J.; Kosuga, T.; Konishi, H.; Shiozaki, A.; et al. Overexpression of PBK/TOPK Relates to Tumour Malignant Potential and Poor Outcome of Gastric Carcinoma. Br. J. Cancer 2017, 116, 218–226.
