The highly aggressive and invasive glioblastoma (GBM) tumour is the most malignant lesion among adult-type diffuse gliomas, representing the most common primary brain tumour in the neuro-oncology practice of adults. With a poor overall prognosis and strong resistance to treatment, this nervous system tumour requires new, innovative treatment. GBM is a polymorphic tumour consisting of an array of stromal cells and various malignant cells contributing to tumour initiation, progression, and treatment response. Cannabinoids possess anti-cancer potencies against glioma cell lines and in animal models. To improve existing treatment, cannabinoids as functionalised ligands on nanocarriers were investigated as potential anti-cancer agents. The GBM tumour microenvironment is a multifaceted system consisting of resident or recruited immune cells, extracellular matrix components, tissue-resident cells, and soluble factors.
- endocannabinoid system
- glioblastoma tumour
- glioblastoma tumour microenvironment
1. Introduction
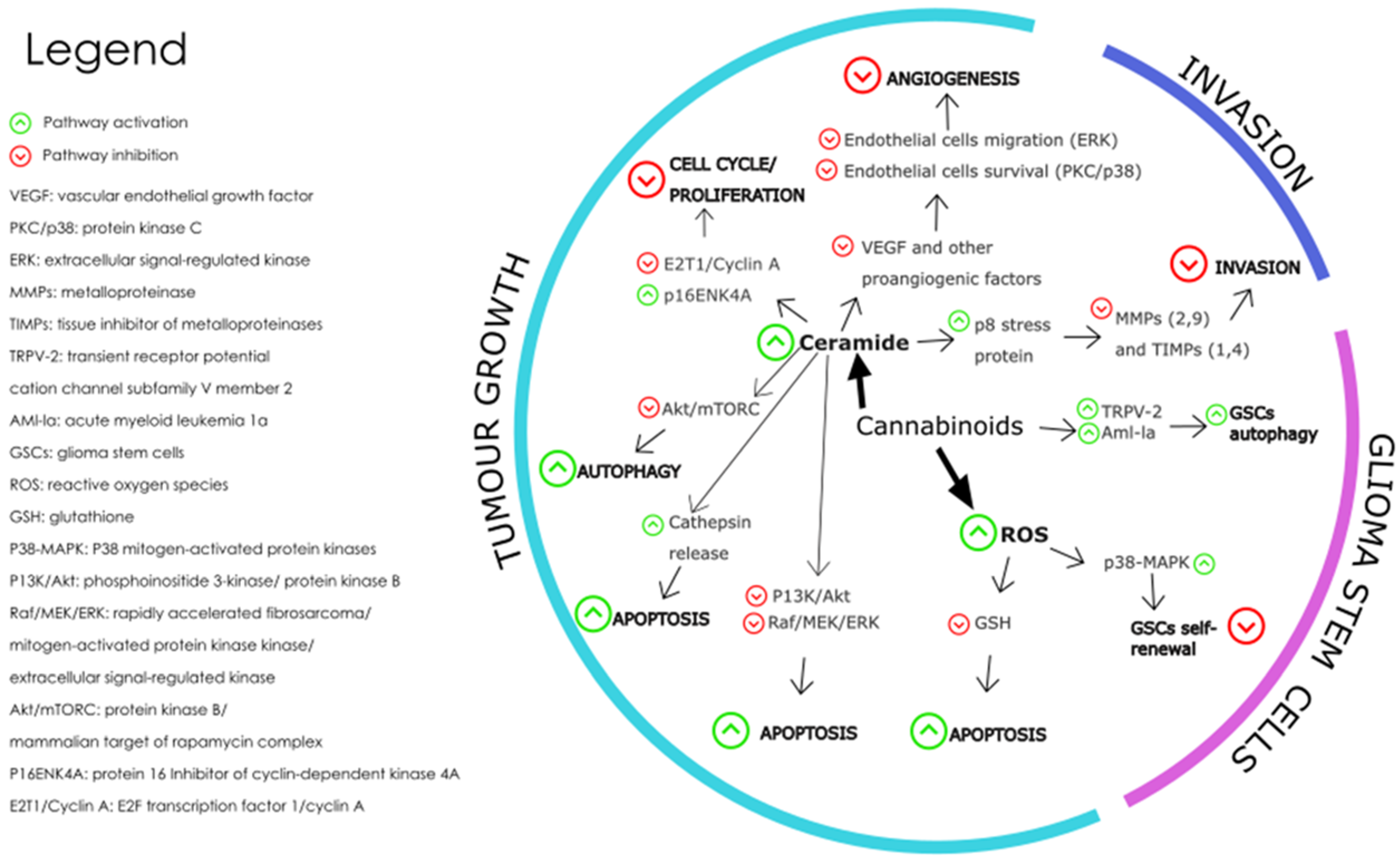
2. Cannabinoids as a Promising Adjuvant in the Treatment of GBM
The endocannabinoid system which includes endocannabinoids and the enzymes that synthesise and degrade them, and the transporters and G-protein coupled receptors involved in their signalling have been found in glioblastoma cells [31,101][31][40]. The ECS is a homeostatic system that uses lipid-derived signalling molecules to regulate a wide range of physiological functions [102][41]. Studies have shown high levels of cannabinoid receptors, CB1-R and CB2-R, as well as the transient receptor potential vanilloid 1 receptor expressed on glioblastoma cells, which are regulated by genetic and epigenetic mechanisms [103][42]. Although the expression levels obtained by immunohistochemistry are heterogeneous and dependent on the age of the patient and the histopathological origin of the brain tumour cells, CB2-R expression has been positively correlated with tumour grade and upregulated in most glioblastomas [104][43]. According to immunohistochemical analysis, both CB1 and CB2 receptors were detected in around 38% and 54% of glioblastoma endothelial cells, respectively [105][44]. CB2-R expression levels were found to be higher than CB1 in glioblastoma tissues. These findings suggest that selective CB2-R agonists could potentially serve as crucial targets for the treatment of glioma. The term “cannabinoids” originally described bioactive constituents of the Cannabis sativa plant. It is now an umbrella term covering a broad range of compounds subsectioned into the synthetic cannabinoids, the phytocannabinoids, and the endogenous cannabinoids, most of which are ligands which bind to endogenous cannabinoid (e.g., CB1-R and CB2-R) and other G-protein coupled receptors [105,106][44][45]. The endogenous cannabinoids are naturally occurring lipid mediators that are synthesised from the membrane phospholipids of cells [107][46]. Table 41 provides an overview of the main classes of cannabinoids: classical cannabinoids, non-classical cannabinoids, aminoalkylindoles, and eicosanoids [108][47]. The table summarises the structural characteristics, formulation strategies, and metabolism for each class. This information can be used to understand the unique properties of each class of cannabinoids.| Classical Cannabinoids |
| Classical cannabinoids are the most well-known group of cannabinoids, and they are found in the cannabis plant. They have a highly lipophilic structure and poor water solubility due to their characteristic tricyclic terpenophenolic structure [109][48]. This lipophilicity facilitates easy passage through the lipid bilayers of cell membranes, influencing their absorption and distribution. Classical cannabinoids are extensively metabolised in the liver, primarily by cytochrome P450 enzymes, leading to a variety of metabolites, some of which are active and contribute to its pharmacological effects [110,111][49][50]. The high lipophilicity and poor water solubility of classical cannabinoids pose challenges in formulating them for aqueous-based delivery systems [112][51]. Techniques like nanoemulsions, liposomes, or microencapsulation may be employed to enhance solubility and bioavailability. Examples: THC, CBD, CBN |
| Non-Classical Cannabinoids |
| Non-classical cannabinoids, often synthetic cannabinoids that are not found in the cannabis plant, can be designed to have specific physicochemical properties [113][52]. They may be more potent and selective for cannabinoid receptors than classical cannabinoids [114][53]. They may be designed to have increased metabolic stability, thereby prolonging their duration of action [115][54]. However, their synthetic nature might lead to unpredictable metabolism and potential toxic metabolites. Formulation strategies would depend on the specific properties of the compound. Solubility enhancement and targeted delivery systems could be key considerations. Examples: CP 47497, CP 55940 |
| Aminoalkylindoles |
| Aminoalkylindoles have a simpler, more stable structure compared to classical cannabinoids. The aminoalkylindole chemical class can be subdivided into four groups: naphthoylindoles, phenylacetylindoles, benzoylindoles, and naphthylmethylindoles [116][55]. This influences their interaction with cannabinoid receptors, making them more selective for cannabinoid receptors [117][56]. These compounds generally have high lipophilicity and may show significant brain penetration due to their ability to cross the blood–brain barrier efficiently. Similar to classical cannabinoids, addressing solubility and stability issues is critical [118][57]. There’s also a need to consider the potential for rapid onset of action due to efficient CNS penetration. Examples: WIN-55212-2, JWH-018 |
| Eicosanoids |
| Endocannabinoids, including anandamide and 2-AG, are derived from fatty acids, making them lipophilic structures [119,120][58][59]. This allows easier cellular uptake and interaction with cannabinoid receptors [121][60]. Endocannabinoids are rapidly metabolised in the body, which can limit their therapeutic use unless modifications or delivery systems are employed to stabilise them [122][61]. Enhancing stability and prolonging the duration of action are primary goals. Techniques might include the use of enzyme inhibitors to prevent rapid degradation or using advanced delivery systems to target specific tissues. Examples: Anandamide, 2-AG |
References
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529.
- Merve, A.; Millner, T.O.; Marino, S. Integrated Phenotype–Genotype Approach in Diagnosis and Classification of Common Central Nervous System Tumours. Histopathology 2019, 75, 299–311.
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. CIMPACT-NOW Update 3: Recommended Diagnostic Criteria for “Diffuse Astrocytic Glioma, IDH-Wildtype, with Molecular Features of Glioblastoma, WHO Grade IV”. Acta Neuropathol. 2018, 136, 805–810.
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A.; Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; et al. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195.
- Perus, L.J.M.; Walsh, L.A. Microenvironmental Heterogeneity in Brain Malignancies. Front. Immunol. 2019, 10, 2294.
- Mukherjee, S.; Pillai, P.P. Current Insights on Extracellular Vesicle-Mediated Glioblastoma Progression: Implications in Drug Resistance and Epithelial-Mesenchymal Transition. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130065.
- Abels, E.R.; Maas, S.L.N.; Tai, E.; Ting, D.T.; Broekman, M.L.D.; Breakefield, X.O.; El Khoury, J. GlioM&M: Web-Based Tool for Studying Circulating and Infiltrating Monocytes and Macrophages in Glioma. Sci. Rep. 2020, 10, 9898.
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional Communication in the Microenvirons of Glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495.
- Allen, B.M.; Hiam, K.J.; Burnett, C.E.; Venida, A.; DeBarge, R.; Tenvooren, I.; Marquez, D.M.; Cho, N.W.; Carmi, Y.; Spitzer, M.H. Systemic Dysfunction and Plasticity of the Immune Macroenvironment in Cancer Models. Nat. Med. 2020, 26, 1125.
- Erdman, S.E.; Poutahidis, T. The Microbiome Modulates the Tumor Macroenvironment. OncoImmunology 2014, 3, e28271.
- Sverdlov, E.D. Multidimensional Complexity of Cancer. Simple Solutions Are Needed. Biochemistry 2016, 81, 731–738.
- Del Bianco, P.; Pinton, L.; Magri, S.; Canè, S.; Masetto, E.; Basso, D.; Padovan, M.; Volpin, F.; d’Avella, D.; Lombardi, G.; et al. Myeloid Diagnostic and Prognostic Markers of Immune Suppression in the Blood of Glioma Patients. Front. Immunol. 2022, 12, 5672.
- Ugel, S.; De Sanctis, F.; Mandruzzato, S.; Bronte, V. Tumor-Induced Myeloid Deviation: When Myeloid-Derived Suppressor Cells Meet Tumor-Associated Macrophages. J. Clin. Investig. 2015, 125, 3365–3376.
- Psaila, B.; Lyden, D. The Metastatic Niche: Adapting the Foreign Soil. Nat. Rev. Cancer 2009, 9, 285–293.
- Lah, T.T.; Novak, M.; Breznik, B. Brain Malignancies: Glioblastoma and Brain Metastases. Semin. Cancer Biol. 2020, 60, 262–273.
- Sceneay, J.; Smyth, M.J.; Möller, A. The Pre-Metastatic Niche: Finding Common Ground. Cancer Metastasis Rev. 2013, 32, 449–464.
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265.
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268.
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615.
- Ueda, N.; Tsuboi, K.; Uyama, T. Metabolism of Endocannabinoids and Related N-Acylethanolamines: Canonical and Alternative Pathways. FEBS J. 2013, 280, 1874–1894.
- Khan, M.I.; Sobocinska, A.A.; Czarnecka, A.M.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and Their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766.
- Contino, M.; McCormick, P.J. Editorial: The Canonical and Non-Canonical Endocannabinoid System as a Target in Cancer and Acute and Chronic Pain. Front. Pharmacol. 2020, 11, 312.
- Biringer, R.G. Endocannabinoid Signaling Pathways: Beyond CB1R and CB2R. J. Cell Commun. Signal. 2021, 15, 335–360.
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734.
- Simard, M.; Archambault, A.S.; Lavoie, J.P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and Metabolism of Endocannabinoids and Their Congeners from the Monoacylglycerol and N-Acyl-Ethanolamine Families. Biochem. Pharmacol. 2022, 205, 115261.
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Gupta, V.; Chitranshi, N.; Godinez, A.; Saks, D.; Hasan, M.; Amirkhani, A.; McKay, M.; et al. A Proteomic View of Cellular and Molecular Effects of Cannabis. Biomolecules 2021, 11, 1411.
- Costas-Insua, C.; Guzmán, M. Endocannabinoid Signaling in Glioma. Glia 2022, 71, 127–138.
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282.
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989.
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Torres-Suárez, A.I. Insights into the Effects of the Endocannabinoid System in Cancer: A Review. Br. J. Pharmacol. 2018, 175, 2566–2580.
- Rocha, F.C.M.; Dos Santos Júnior, J.G.; Stefano, S.C.; Da Silveira, D.X. Systematic Review of the Literature on Clinical and Experimental Trials on the Antitumor Effects of Cannabinoids in Gliomas. J. Neurooncol. 2014, 116, 11–24.
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of Endocannabinoid System in Human Gliomas. J. Neurochem. 2012, 120, 842–849.
- De Jesús, M.L.; Hostalot, C.; Garibi, J.M.; Sallés, J.; Meana, J.J.; Callado, L.F. Opposite Changes in Cannabinoid CB1 and CB2 Receptor Expression in Human Gliomas. Neurochem. Int. 2010, 56, 829–833.
- Held-Feindt, J.; Dörner, L.; Sahan, G.; Mehdorn, H.M.; Mentlein, R. Cannabinoid Receptors in Human Astroglial Tumors. J. Neurochem. 2006, 98, 886–893.
- Sredni, S.T.; Huang, C.C.; Suzuki, M.; Pundy, T.; Chou, P.; Tomita, T. Spontaneous Involution of Pediatric Low-Grade Gliomas: High Expression of Cannabinoid Receptor 1 (CNR1) at the Time of Diagnosis May Indicate Involvement of the Endocannabinoid System. Child’s Nerv. Syst. 2016, 32, 2061–2067.
- Carracedo, A.; Lorente, M.; Egia, A.; Blázquez, C.; García, S.; Giroux, V.; Malicet, C.; Villuendas, R.; Gironella, M.; González-Feria, L.; et al. The Stress-Regulated Protein P8 Mediates Cannabinoid-Induced Apoptosis of Tumor Cells. Cancer Cell 2006, 9, 301–312.
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of Cannabinoid Delivery: How Can Nanomedicine Help? Nanomedicine 2020, 15, 2023–2028.
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle Drones to Target Lung Cancer with Radiosensitizers and Cannabinoids. Front. Oncol. 2017, 7, 208.
- Deshpande, A.; Patil, T.S. Nanocarrier Technologies for Enhancing the Solubility and Dissolution Rate of Api. In Medicinal Chemistry with Pharmaceutical Product Development; Apple Academic Press: Cambridge, MA, USA, 2019; pp. 155–234.
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845.
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279.
- Doherty, G.J.; de Paula, B.H.R. Cannabinoids in Glioblastoma Multiforme—Hype or Hope? Br. J. Cancer 2021, 124, 1341–1343.
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive Pattern of Cannabinoid Receptor Type II (CB2) Expression in Adult and Pediatric Brain Tumors. Brain Res. 2007, 1137, 161–169.
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852.
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19.
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of Inflammation by Cannabinoids, the Endocannabinoids 2-Arachidonoyl-Glycerol and Arachidonoyl-Ethanolamide, and Their Metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070.
- Shevyrin, V.A.; Morzherin, Y.Y. Cannabinoids: Structures, Effects, and Classification. Russ. Chem. Bull. 2015, 64, 1249–1266.
- Bow, E.W.; Rimoldi, J.M. The Structure–Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect. Med. Chem. 2016, 8, 17.
- Su, M.K.; Seely, K.A.; Moran, J.H.; Hoffman, R.S. Metabolism of Classical Cannabinoids and the Synthetic Cannabinoid JWH-018. Clin. Pharmacol. Ther. 2015, 97, 562–564.
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and Drug Metabolizing Enzymes: Potential for Drug-Drug Interactions and Implications for Drug Safety and Efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460.
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557.
- Bloom, A.S.; Edgemond, W.S.; Moldvan, J.C. Nonclassical and Endogenous Cannabinoids: Effects on the Ordering of Brain Membranes. Neurochem. Res. 1997, 22, 563–568.
- Pop, E. Cannabinoids, Endogenous Ligands and Synthetic Analogs. Curr. Opin. Chem. Biol. 1999, 3, 418–425.
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226.
- Shevyrin, V.; Melkozerov, V.; Endres, G.W.; Shafran, Y.; Morzherin, Y. On a New Cannabinoid Classification System: A Sight on the Illegal Market of Novel Psychoactive Substances. Cannabis Cannabinoid Res. 2016, 1, 186–194.
- Shim, J.-Y.; Collantes, E.R.; Welsh, W.J.; Howlett, A.C. Unified Pharmacophoric Model for Cannabinoids and Aminoalkylindoles. In Molecular Modeling and Prediction of Bioactivity; Springer: Boston, MA, USA, 2000; pp. 201–206.
- Mardal, M.; Gracia-Lor, E.; Leibnitz, S.; Castiglioni, S.; Meyer, M.R. Toxicokinetics of New Psychoactive Substances: Plasma Protein Binding, Metabolic Stability, and Human Phase I Metabolism of the Synthetic Cannabinoid WIN 55,212-2 Studied Using in Vitro Tools and LC-HR-MS/MS. Drug Test. Anal. 2016, 8, 1039–1048.
- Burstein, S.H. Eicosanoid Mediation of Cannabinoid Actions. Bioorg. Med. Chem. 2019, 27, 2718–2728.
- Burstein, S.H.; Zurier, R.B. Cannabinoids, Endocannabinoids, and Related Analogs in Inflammation. AAPS J. 2009, 11, 109–119.
- Deutsch, D.G. A Personal Retrospective: Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs). Front. Pharmacol. 2016, 7, 370.
- Rouzer, C.A.; Ghebreselasie, K.; Marnett, L.J. Chemical Stability of 2-Arachidonylglycerol under Biological Conditions. Chem. Phys. Lipids 2002, 119, 69–82.
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747.
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Cannabinoid Signaling in Glioma Cells. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2013.
- Downer, E.J.; Gowran, A.; Murphy, Á.C.; Campbell, V.A. The Tumour Suppressor Protein, P53, Is Involved in the Activation of the Apoptotic Cascade by Δ9-Tetrahydrocannabinol in Cultured Cortical Neurons. Eur. J. Pharmacol. 2007, 564, 57–65.
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid Receptor Agonist-Induced Apoptosis of Human Prostate Cancer Cells LNCaP Proceeds through Sustained Activation of ERK1/2 Leading to G 1 Cell Cycle Arrest. J. Biol. Chem. 2006, 281, 39480–39491.
- Galanti, G.; Fisher, T.; Kventsel, I.; Shoham, J.; Gallily, R.; Mechoulam, R.; Lavie, G.; Amariglio, N.; Rechavi, G.; Toren, A. Δ9-Tetrahydrocannabinol Inhibits Cell Cycle Progression by Downregulation of E2F1 in Human Glioblastoma Multiforme Cells. Acta. Oncol. 2008, 47, 1062–1070.
- Irrera, N.; D’ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038.
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623.
- O’Reilly, E.M.; Cosgrave, J.M.; Gallagher, W.M.; Perry, A.S. Plant-Derived Cannabinoids as Anticancer Agents. Trends Cancer 2022, 8, 350–357.
- Hinz, B.; Ramer, R. Cannabinoids as Anticancer Drugs: Current Status of Preclinical Research. Br. J. Cancer 2022, 127, 1–13.
- Kolbe, M.R.; Hohmann, T.; Hohmann, U.; Ghadban, C.; Mackie, K.; Zöller, C.; Prell, J.; Illert, J.; Strauss, C.; Dehghani, F. THC Reduces Ki67-Immunoreactive Cells Derived from Human Primary Glioblastoma in a GPR55-Dependent Manner. Cancers 2021, 13, 1064.
- Oesch, S.; Gertsch, J. Cannabinoid Receptor Ligands as Potential Anticancer Agents—High Hopes for New Therapies? J. Pharm. Pharmacol. 2010, 61, 839–853.
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the Landscape of Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534.
- Cherkasova, V.; Wang, B.; Gerasymchuk, M.; Fiselier, A.; Kovalchuk, O.; Kovalchuk, I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers 2022, 14, 5142.
- Worster, B.; Hajjar, E.R.; Handley, N. Cannabis Use in Patients with Cancer: A Clinical Review. JCO Oncol. Pract. 2022, 18, 743–749.
- Klimkiewicz, A.; Jasinska, A. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Psychiatria 2017, 15, 88–92.
- Sánchez, C.; Galve-Roperh, I.; Canova, C.; Brachet, P.; Guzmán, M. Δ9-Tetrahydrocannabinol Induces Apoptosis in C6 Glioma Cells. FEBS Lett. 1998, 436, 6–10.
- Allister, S.D.; Chan, C.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids Selectively Inhibit Proliferation and Induce Death of Cultured Human Glioblastoma Multiforme Cells. J. Neurooncol. 2005, 74, 31–40.
- End, D.W.; Thoursen, K.; Dewey, W.L.; Carchman, R.A. A Comparative Study of the Disposition of Tetrahydrocannabinol in Neuroblastoma and Glioma Cells in Tissue Culture: Relation to Cellular Impairment. Mol. Pharmacol. 1977, 13, 864–871.
- Velasco, G.; Galve-Roperh, I.; Sánchez, C.; Blázquez, C.; Guzmán, M. Hypothesis: Cannabinoid Therapy for the Treatment of Gliomas? Neuropharmacology 2004, 47, 315–323.
- Bifulco, M.; Laezza, C.; Gazzerro, P.; Pentimalli, F. Endocannabinoids as Emerging Suppressors of Angiogenesis and Tumor Invasion (Review). Oncol. Rep. 2007, 17, 813–816.
- Carkaci-Salli, N.; Raup-Konsavage, W.M.; Karelia, D.; Sun, D.; Jiang, C.; Lu, J.; Vrana, K.E. Cannabinoids as Potential Cancer Therapeutics: The Concentration Conundrum. Cannabis Cannabinoid Res. 2023.
- Jo, Y.; Choi, N.; Kim, K.; Koo, H.J.; Choi, J.; Kim, H.N. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-Mimicking in Vitro Models in Anti-Cancer Drug Development. Theranostics 2018, 8, 5259–5275.
- Fisher, T.; Golan, H.; Schiby, G.; Prichen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In Vitro and in Vivo Efficacy of Non-Psychoactive Cannabidiol in Neuroblastoma. Curr. Oncol. 2016, 23, S15–S22.
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159.
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Investig. 2009, 119, 1359–1372.
- Ciechomska, I.A.; Gabrusiewicz, K.; Szczepankiewicz, A.A.; Kaminska, B. Endoplasmic Reticulum Stress Triggers Autophagy in Malignant Glioma Cells Undergoing Cyclosporine A-Induced Cell Death. Oncogene 2012, 32, 1518–1529.
- Massi, P.; Valenti, M.; Solinas, M.; Parolaro, D. Molecular Mechanisms Involved in the Antitumor Activity of Cannabinoids on Gliomas: Role for Oxidative Stress. Cancers 2010, 2, 1013–1026.
- Wang, K.; Wang, Q.; Li, Q.; Zhang, Z.; Gao, J.; Fan, C.; Sun, B.; Ni, Q. Cannabinoid WIN 55,212-2 Inhibits Human Glioma Cell Growth by Triggering ROS-Mediated Signal Pathways. BioMed Res. Int. 2021, 2021, 6612592.
- Blázquez, C.; Casanova, M.L.; Planas, A.; Gómez del Pulgar, T.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragonés, J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 2003, 17, 529–531.
- Widmer, M.; Hanemann, C.O.; Zajicek, J. High Concentrations of Cannabinoids Activate Apoptosis in Human U373MG Glioma Cells. J. Neurosci. Res. 2008, 86, 3212–3220.
- Peeri, H.; Shalev, N.; Vinayaka, A.C.; Nizar, R.; Kazimirsky, G.; Namdar, D.; Anil, S.M.; Belausov, E.; Brodie, C.; Koltai, H. Specific Compositions of Cannabis Sativa Compounds Have Cytotoxic Activity and Inhibit Motility and Colony Formation of Human Glioblastoma Cells In Vitro. Cancers 2021, 13, 1720.
- De los Reyes Corrales, T.; Losada-Pérez, M.; Casas-Tintó, S. JNK Pathway in CNS Pathologies. Int. J. Mol. Sci. 2021, 22, 3883.
- Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. Cannabinoids as Modulators of Cell Death: Clinical Applications and Future Directions; Springer: Cham, Switzerland, 2017; pp. 63–88.
- Rodriguez-Almaraz, J.E.; Butowski, N. Therapeutic and Supportive Effects of Cannabinoids in Patients with Brain Tumors (CBD Oil and Cannabis). Curr. Treat. Options Oncol. 2023, 24, 30–44.
- Ganesh, A.N.; McLaughlin, C.K.; Duan, D.; Shoichet, B.K.; Shoichet, M.S. A New Spin on Antibody-Drug Conjugates: Trastuzumab-Fulvestrant Colloidal Drug Aggregates Target HER2-Positive Cells. ACS Appl. Mater. Interfaces 2017, 9, 12195–12202.
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A Pilot Clinical Study of Δ9-Tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 2006, 95, 197–203.
- Johnson, E.C.; Hatoum, A.S.; Deak, J.D.; Polimanti, R.; Murray, R.M.; Edenberg, H.J.; Gelernter, J.; Di Forti, M.; Agrawal, A. The Relationship between Cannabis and Schizophrenia: A Genetically Informed Perspective. Addiction 2021, 116, 3227–3234.
- Marijuana and Madness; Castle, D.; Murray, R.M.; D’Souza, D.C. (Eds.) Cambridge University Press: Cambridge, UK, 2011.
- Hindley, G.; Beck, K.; Borgan, F.; Ginestet, C.E.; McCutcheon, R.; Kleinloog, D.; Ganesh, S.; Radhakrishnan, R.; D’Souza, D.C.; Howes, O.D. Psychiatric Symptoms Caused by Cannabis Constituents: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2020, 7, 344–353.
- Larsen, C.; Shahinas, J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J. Clin. Med. Res. 2020, 12, 129–141.
- Sawtelle, L.; Holle, L.M. Use of Cannabis and Cannabinoids in Patients with Cancer. Ann. Pharmacother. 2021, 55, 870–890.
- Hostiuc, S.; Moldoveanu, A.; Negoi, I.; Drima, E. The Association of Unfavorable Traffic Events and Cannabis Usage: A Meta-Analysis. Front. Pharmacol. 2018, 9, 99.
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug–Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989.
- Buchtova, T.; Lukac, D.; Skrott, Z.; Chroma, K.; Bartek, J.; Mistrik, M. Drug–Drug Interactions of Cannabidiol with Standard-of-Care Chemotherapeutics. Int. J. Mol. Sci. 2023, 24, 2885.
