Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Narayanan Sadagopan.
Patients with advanced hepatocellular carcinoma (HCC) have several systemic treatment options. Conventional chemotherapy is almost never used for advanced-stage disease, which instead is treated with immunotherapy, tyrosine kinase inhibitors, and VEGF inhibitors. Immune checkpoint inhibitors targeting various receptors have been or are currently undergoing clinical evaluation. Ongoing trials with three-drug regimens may be the future of advanced-stage HCC treatment. Other immune-modulatory approaches of chimeric antigen receptor-modified T cells, bispecific antibodies, cytokine-induced killer cells, natural killer cells, and vaccines are in early-stage clinical trials.
- hepatocellular carcinoma
- immunotherapy
- VEGF
- tyrosine kinase inhibitors
1. Introduction
Hepatocellular carcinoma (HCC) is not a conventional solid tumor malignancy. Traditional systemic chemotherapy drugs are not very effective, possibly due to higher expression of drug resistance genes and due to baseline liver dysfunction [1]. Unlike most malignancies, HCC can be diagnosed without a tissue biopsy and just with imaging. A four-phase CT scan of the abdomen with IV contrast or a liver MRI with contrast can diagnose HCC in patients with cirrhosis or chronic hepatitis B [2]. A four-phase CT scan can have a sensitivity as high as 90% for HCC greater than 2 cm in diameter [3]. While this can initially benefit the patient by avoiding an invasive diagnostic procedure, modern cancer work-up often involves sending tissue samples for next-generation sequencing to look for targetable mutations or markers of responsiveness to treatment. Limited tissue sampling may have hampered some advancements in HCC therapy.
Treatment, as always in cancer, begins with staging, although in this respect HCC is once again unique. The Barcelona Clinic Liver Cancer (BCLC) staging system is the most used approach. It incorporates Eastern Cooperative Oncology Group (ECOG) performance status; Child–Pugh class, which is an estimate of mortality in cirrhosis patients; size and number of tumors; vascular involvement; and extrahepatic spreading of the tumor [4]. Barcelona stage B (intermediate stage) or stage C (advanced stage) patients may be eligible for systemic treatment, which is the focus of this revisewarch. Due to the ineffectiveness of chemotherapy, most of the current approved systemic treatments for HCC are tyrosine kinase inhibitors (TKI), vascular endothelial growth factor (VEGF) inhibitors, and immunotherapy agents [5].
2. Evolution of First-Line Treatment
In 2007, the TKI sorafenib was the first systemic therapy to show benefits for advanced HCC with a median overall survival (OS) of 10.7 months compared to 7.9 months for placebo [11][6]. In 2018, the TKI lenvatinib was shown to be non-inferior to sorafenib in the REFLECT trial, which was an open-label phase III trial of 954 patients. The median OS of patients treated with lenvatinib was 13.6 months vs. 12.3 months for sorafenib [12][7]. The landmark IMbrave150 trial published in 2020 switched the initial systemic treatment approach for advanced HCC away from TKIs towards immunotherapy. Atezolizumab plus bevacizumab provided a significantly better median OS than sorafenib (19.2 months vs. 13.4 months) and significantly improved progression-free survival (PFS: 6.9 months vs. 4.3 months). Treatment-related grade 3 or 4 adverse events were similar between groups. No patients had grade 3 or 4 pneumonitis or myocarditis, which are among the more significant immune-related adverse events in patients receiving immunotherapy [13,14][8][9]. These results established atezolizumab plus bevacizumab as one of the preferred first-line regimens at this time. The other preferred first-line regimen is the immunotherapy doublet tremelimumab plus durvalumab, which came out of the HIMALAYA trial published in 2022. Of note, patients in the tremelimumab plus durvalumab group only received one total dose of tremelimumab each. Tremelimumab plus durvalumab had a median OS of 16.4 months compared to 13.8 months for sorafenib (p = 0.0035) [15][10]. It is interesting to see the median OS of sorafenib increasing in more recent trials, pointing to a generalized improvement in cancer care (Figure 21).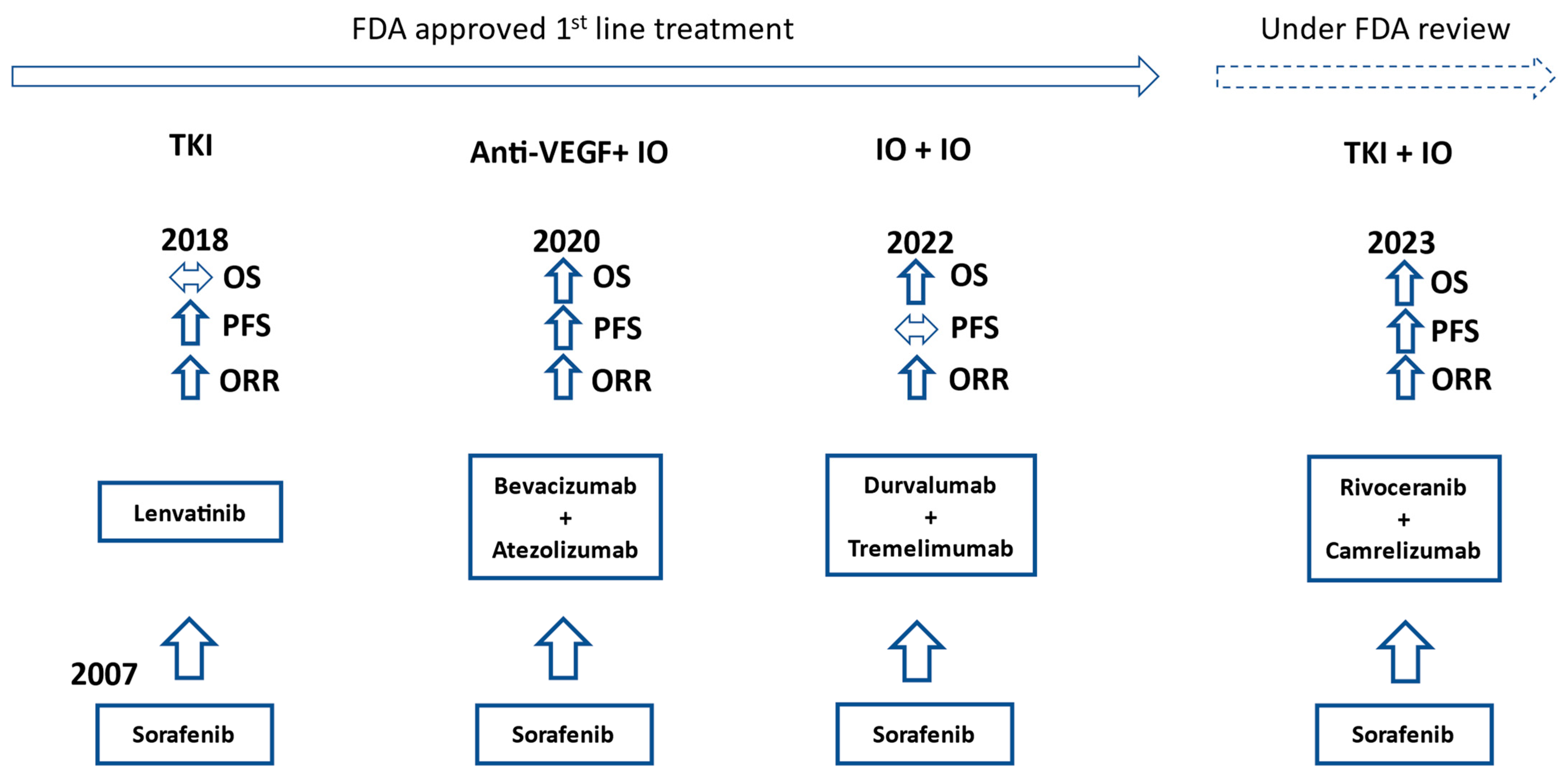
Figure 21.
Timeline of FDA approvals for first-line advanced HCC treatment. OS, PFS, and ORR comparisons to sorafenib in their respective trials. IO = immunotherapy.
3. Second-Line and Beyond
There are an increasing number of approved first-line treatment options, while all second-line treatment options were tested in patients who received first-line sorafenib treatment. There is no guideline on how to prioritize and sequence all the treatment options. In practice, many of the first-line options are used in the later-line setting, assuming the patient has not received those agents yet. This pushes the approved second-line treatment into later lines of therapy if patients are still in good shape to receive these treatments. The approved second-line and beyond options are included in Table 1. Regorafenib and cabozantinib are both mixed TKIs, and their common side effects are hypertension, hand-foot syndrome, fatigue, and diarrhea [20,21][15][16]. Ramucirumab, a monoclonal antibody to VEGFR2, was explicitly approved for patients with alpha-fetoprotein (AFP) greater than 400 ng/mL [22][17]. KEYNOTE-240 and KEYNOTE-394 both looked at pembrolizumab in the second-line setting. However, KEYNOTE-394 involved only patients in Asia, while KEYNOTE-240 studied a more diverse population [23,24][18][19]. The multi-arm phase I/II study CheckMate 040 compared different dosing regimens of nivolumab and ipilimumab without a control arm. The 22.8-month median OS was from the group who received nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for 4 cycles. This regimen was followed by nivolumab 240 mg every 2 weeks. That group also had an ORR of 32% [25][20]. It should be noted that all the trials mentioned in Table 1 are based on patients having progression on sorafenib, which is currently not the first-line standard of care.Table 1. Trial results leading to approval of later-line options for advanced HCC [20,21,22,23,24,25].
| Drug (Trial) | Control | Total Number of Patients | Drug Median OS (Months) | Control Median OS (Months) | HR (95% CI) |
|---|---|---|---|---|---|
| Regorafenib (RESORCE) |
placebo | 573 | 10.6 | 7.8 | 0.63 (0.5–0.79) |
| Ramucirumab (REACH-2) |
placebo | 292 | 8.5 | 7.3 | 0.71 (0.53–0.95) |
| Cabozantinib (CELESTIAL) |
placebo | 707 | 10.2 | 8 | 0.76 (0.63–0.92) |
| Pembrolizumab (KEYNOTE-240) | placebo | 413 | 13.9 | 10.6 | 0.78 (0.61–0.998) |
| Pembrolizumab (KETNOTE-394) | placebo | 453 | 14.6 | 13 | 0.79 (0.63–0.99) |
| Nivolumab + Ipilimumab (CheckMate 040) |
n/a | 148 | 22.8 | n/a | n/a |
4. Completed Phase III Trials
With immunotherapy and TKIs independently showing success in HCC, the combination of the 2 have been evaluated in many phase III HCC clinical trials. VEGF, in addition to its potent role in angiogenesis, is also an immunosuppressive factor, so blocking this factor with a TKI could potentiate the effect of immunotherapy [26][21]. Camrelizumab is an PD-1 antibody, while rivoceranib (also known as apatinib) is a VEGFR-2 targeting TKI. That combination was compared to sorafenib in the first-line setting in a trial involving 543 patients. Median PFS was 5.6 months for the 2 drugs vs. 3.7 months for sorafenib (HR 0.52, 95% CI 0.41–0.65) and median OS was 22.1 months for the 2 agents vs. 15.2 months for sorafenib (HR 0.62 and 95% CI 0.49–0.8). The side effect profile was as expected for the camrelizumab plus rivoceranib group, with the most common grade 3 or 4 adverse effects being hypertension (38%), increase in aspartate aminotransferase (17%), increase in alanine aminotransferase (13%), and hand-foot syndrome (12%). The trial population was about 83% Asian, about 75% HCC due to hepatitis B, and only Child–Pugh class A, which limits its generalizability somewhat [27][22]. Nonetheless, camrelizumab plus rivoceranib may soon be another first-line HCC treatment option. The COSMIC-312 trial looked at a combination of 2 approved agents, atezolizumab and cabozantinib. The trial was conducted in the first-line setting with patients who were Child–Pugh class A. The 3 groups consisted of cabozantinib plus atezolizumab, sorafenib, and cabozantinib. The study showed a median PFS of 6.8 months for the combination group compared to 4.2 months for sorafenib (HR 0.63 and 99% CI 0.44–0.91). The initial reported median OS was not different at 15.4 months for the combination compared to 15.5 months for sorafenib. It was noted that the patients in the sorafenib arm received more subsequent lines of treatment versus the combined group, which likely played a role in the median OS result of the control arm. Final longer-term OS data is pending for this trial. Hypertension, increased AST/ALT, and hand-foot syndrome were again the most common serious adverse events [28][23]. Sintilimab, a PD-1 inhibitor, with IBI305, a bevacizumab biosimilar, was approved in China as a first-line HCC treatment after favorable results from the ORIENT-32 trial. In this trial, a combination of sintilimab plus IBI305 was compared to sorafenib in a trial population of 595 patients. The 2-drug regimen significantly improved the median PFS (4.6 months vs. 2.8 months) and OS (median not reached vs. 10.4 months). This trial took place in 50 clinical sites in China, and 94% of the patients had HCC due to hepatitis B [29][24]. Fundamentally, this regimen is more or less the same as atezolizumab plus bevacizumab, with the only difference being the target on the PD-1-PD-L1 axis. Atezolizumab targets PD-L1. Tislelizumab, an anti-PD-1 monoclonal antibody, was compared to sorafenib in the phase III RATIONALE-301 trial in the first-line setting [30][25]. Tislelizumab was designed to minimize binding to Fc gamma receptors on macrophages to prevent macrophage-mediated destruction of T cells [31][26]. Tislelizumab was found to have non-inferior median OS compared to sorafenib (15.9 months vs. 14.1 months, HR 0.85, and 95% CI 0.71–1.02). Tislelizumab had a higher ORR of 14.3% compared to 5.4%. Tislelizumab had lower rates of grade 3 and 4 adverse events as well as a lower rate of adverse events leading to drug discontinuation. Hepatitis and hypothyroidism were the most common immune-mediated side effects, both at 5.3% [30][25]. ADI-PEG 20 was a different HCC treatment approach in the second-line setting. ADI-PEG 20 is a cloned arginine-degrading enzyme. HCC typically lacks argininosuccinate synthetase, which is required to metabolize citrulline to arginine. With HCC cells being unable to produce arginine and with external arginine being broken down by ADI-PEG 20, the tumor cells would not have access to arginine. In a study of 635 patients, ADI-PEG 20 was compared to placebo; there was no significant difference in median OS (7.8 months vs. 7.4 months) or PFS (2.6 months vs. 2.6 months) [32][27]. Although ADI-PEG 20 did not show any survival benefit over placebo, its tolerable safety profile warranted further investigation in HCC patients with high arginine levels [33][28].References
- Nagahama, H.; Okada, S.; Okusaka, T.; Ishii, H.; Ikeda, M.; Nakasuka, H.; Yoshimori, M. Predictive factors for tumor response to systemic chemotherapy in patients with hepatocellular carcinoma. Jpn. J. Clin. Oncol. 1997, 27, 321–324.
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830.
- Vietti Violi, N.; Lewis, S.; Hectors, S.; Said, D.; Taouli, B. Radiological Diagnosis and Characterization of HCC. In Hepatocellular Carcinoma: Translational Precision Medicine Approaches, 1st ed.; Hoshida, Y., Ed.; Humana Press: Totowa, NJ, USA, 2019; Chapter 4. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553760/ (accessed on 23 August 2023).
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693.
- NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines) Hepatocellular Carcinoma Version 2. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf (accessed on 1 November 2023).
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873.
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Dao, T.V.; De Toni, E.N.; et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022, 1, 8.
- Verset, G.; Borbath, I.; Karwal, M.; Verslype, C.; Van Vlierberghe, H.; Kardosh, A.; Zagonel, V.; Stal, P.; Sarker, D.; Palmer, D.H.; et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin. Cancer Res. 2022, 28, 2547–2554.
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.Y.; Ren, Z.; et al. LBA34—Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S808–S869.
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90.
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 75, 600–609.
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66.
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63.
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296.
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202.
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383.
- Yau, T.; Kang, Y.; Kim, T.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564.
- Bourhis, M.; Palle, J.; Galy-Fauroux, I.; Terme, M. Direct and Indirect Modulation of T Cells by VEGF-A Counteracted by Anti-Angiogenic Treatment. Front. Immunol. 2021, 12, 616837.
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146.
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008.
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990.
- Qin, S.; Kudo, M.; Meyer, T.; Finn, R.S.; Vogel, A.; Bai, Y.; Guo, Y.; Meng, Z.; Zhang, T.; Satoh, T.; et al. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33, S1402–S1403.
- Tislelizumab. Available online: https://www.beigene.com/our-science-and-medicines/tislelizumab/ (accessed on 23 August 2023).
- Abou-Alfa, G.K.; Qin, S.; Ryoo, B.Y.; Lu, S.N.; Yen, C.J.; Feng, Y.H.; Lim, H.Y.; Izzo, F.; Colombo, M.; Sarker, D.; et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018, 29, 1402–1408.
- Study of ADI-PEG 20 versus Placebo in Subjects with High Arginine Level and Unresectable Hepatocellular Carcinoma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05317819 (accessed on 24 August 2023).
More
