The main recent change observed in the field of critical patient infection has been the universal awareness of the need to make better use of antintry summarizes the phenomenon of the emergence of antibacterial resistance and try to share some of the ideas and organizational, microbials, especially in theological, pharmacological, and knowledge tools that scholars consider most seriously ill patients, beyond the application of simple formulas and rigid protocolsuseful and effective for individualized decision making that considers the current context of multidrug resistance. The greatest challenge, therefore, of decision making in this context lies in determining an effective, optimal, and balanced empirical antibiotic treatment. This is sepsis stewardship.
- Antimicrobial Stewardship
- sparing carbapenems
- new antibiotics
- antimicrobial resistance
- individualization
1. Introduction
2. The Current Problem of Antimicrobial Resistance
Antimicrobial resistance is currently one of the greatest challenges in the treatment of critically ill patients. One of the main causes of antimicrobial resistance is the irresponsible use and overuse of antibiotics in livestock, agriculture, the food system, and the health system. This conclusion has been promoted by the One Health initiative created by the World Health Organization (WHO) (https://www.who.int/europe/initiatives/one-health (accessed on 15 January 2024)), in which antimicrobial resistance is considered the most relevant public health emergency [4][5]. In 2019, the number of deaths associated with antimicrobial resistance was estimated to be 4.95 million globally, of which a total of 1.27 million were directly attributed to antimicrobial resistance, and it is estimated that 10 million deaths could be reached by 2050. While the incidence of antimicrobial resistance among Gram-positive bacteria (GPB) appears to be stabilizing, or even decreasing, according to a recent study conducted on patients with bacteremia, the incidence of resistance to Gram-negative bacteria (GNB) is increasing and is currently responsible for the majority of cases and deaths attributable to antimicrobial resistance [6]. However, the fact that the existence of an infection caused by a multidrug-resistant bacterium increases mortality is controversial. The effect of multidrug resistance on costs and length of hospital stay seems clear, but a strict causal effect on mortality, despite a clear association, is debatable. The existence of uncontrolled confounding factors may have led to the determination of such an association in published studies. These pieces of data make antimicrobial resistance a major problem that requires a coordinated global action plan. The WHO has established a Global Action Plan for the Management of Antibiotic Resistance (GAP-AMR) and the creation of a Global Antimicrobial Resistance and Use Surveillance System to achieve the objectives of the program. The main individual risk factors are previous exposure to antibiotics in the last 90 days, hospitalization in the previous 3 months, prolonged current hospitalization greater than 5 days, the presence of invasive devices, and previous colonization or infection by multidrug-resistant drugs. A local epidemiology with high rates of multidrug resistance is an important risk factor that needs to be taken into account [7]. The severity of the illness as a risk factor for multidrug resistance is controversial although the presence of septic shock is a recognized risk factor for multidrug-resistant pneumonia in nosocomial pneumonia and ventilator-associated pneumonia in both European and American guidelines [8]. In a recent multicenter study that included 2621 critically ill patients with intra-abdominal infection, there were no differences in the incidence of multidrug resistance between groups of patients with infections with or without sepsis and those with septic shock [9].3. Antimicrobial Stewardship (AMS)
The goal of AMS is the correct and effective administration of antibiotics, thus minimizing the emergence of resistance, ensuring sustainable use by optimizing the use of antimicrobials in empirical treatment, and refining targeted treatment [10]. However, in a broad sense, what this type of program should pursue is the creation of a “culture of infection” led by a few people who exercise such leadership in a non-coercive way and who decisively influence the rapid diagnosis and correct prescription of antimicrobials based on continuous training and equipment. With a multidisciplinary operating philosophy, this program is not limited to the creation of protocols or to the members of the unit. The existing recommendations regarding who should be part of the responsible group advise that the group should be made up of experts or those more specialized in infection within the ICU, microbiologists with a special vocation for the critically ill patient, and, optionally and according to the hospital’s model, pharmacologists and doctors with special dedication to infection regardless of their specialty [11].3.1. Start Smart
This model of approach to severe infection in critically ill patients encompasses both the optimization of diagnosis and the form of administration and de-escalation strategies, all under the umbrella of AMS programs. In this context, the existence of new biomarkers and rapid microbiological techniques is a key element in this new approach.3.2. Start Smart and then Focus
A few years ago, the government of the United Kingdom promoted the slogan “Start Smart and then focus” (https://www.gov.uk/government/publications/antimicrobial-stewardship-start-smart-then-focus) (accessed on 17 January 2024), and this slogan can be a good model for an approach to the antibiotic treatment of sepsis since the intelligent administration of antibiotics is closely related to a personalized approach, which does not deal with the treatment of all very serious cases in the same way, e.g., a patient in a situation of shock and with a high bacterial load (in which the slogan is “hit fast and strong!”) will not be treated in the same manner as a less severe case who presents a moderate suspicion of infection, even when both may be admitted to the ICU for similar reasons [12]. Key takeaways from a smart start to antimicrobial therapy include-
Only start antimicrobial therapy if there is clear suspicion or evidence of infection;
-
Have a complete history of drug allergies and other patient considerations;
3.3. “… And Then Focus”: What Does This Consist of
The second part of this motto reminds us of the imperative need to review the clinical diagnosis and consider the possibility of changing, de-escalating, or even escalating treatment. Thanks to current advances in rapid microbiological diagnosis, this review may be possible in less than 48 h, allowing decisions to be made about the need to continue antibiotic treatment with the same or other antibiotics, and at what dose, and to document a clear plan of action on the decision to prescribe these antibiotics [13]. Every day, it is necessary to question whether the antibiotic is adequate, whether the dose is optimal, and whether the patient needs it [14]. The Challenge of Treating Superbugs: What to Think About if Antibiotic Treatment Fails-
Delay in administration;
-
Inappropriate spectrum of activity;
-
Initiate early and effective treatment in patients with sepsis and/or life-threatening infections;
-
Inadequate blood levels or inadequate penetration into the focus of the infection;Avoid the widespread use of broad-spectrum antibiotics, without paying attention to accompanying clinical symptoms, when treating any suspected infection;
-
Presence of antibiotic neutralizers and/or antagonists;Be up-to-date and always keep in mind the local microbiology and the patterns of resistance prevalent in the unit, the hospital, and the environment;
-
Presence of superbugs;Obtain cultures before starting therapy when possible, taking into account that treatment should not be too greatly delayed;
-
Presence of a superinfection;In critically ill patients with septic shock, use the best antimicrobial alternative available to resolve the infection, in addition to controlling the outbreak whenever feasible, to reduce the bacterial load as soon as possible [12].
- Non-infectious source of fever;
- The focus of the infection has not been properly controlled.
4. Microbiology: Key Concepts in Sepsis
4.1. Specimens and Diagnostic Techniques
A good microbiological diagnosis must begin with a good choice of the sample provided to the laboratory; this sample should be representative of the suspected infection and its processing should observe correct standards of collection, shipment, documentation, and storage. Microbiological culture continues to be the reference method as it not only makes it possible to characterize the germs causing the infection but also allows the relevant antimicrobial susceptibility tests to be carried out on the sample. However, the need to obtain results quickly and technological advances have led to the development of molecular techniques that are revolutionizing microbiological diagnostics [15]. Among the rapid techniques, two currently stand out:- -
-
MALDI-TOF (matrix-assisted laser desorption ionization–time of flight): a technique based on mass spectrometry. MALDI-TOF currently allows the identification of microorganisms in less than 30 min (previously, it took up to 18 h) through the analysis of their protein profile. It is perhaps the most widespread rapid technique [16].
- -
-
RT-PCR (real-time polymerase chain reaction): a molecular technique that allows the direct identification of germs from the sample.
4.2. Minimum Inhibitory Concentration (MIC)
When performing an in vitro antibiotic susceptibility technique, the main objective is to predict whether a patient with an infection by a certain germ can be treated with an antimicrobial or if another option should be chosen. Minimum inhibitory concentration (MIC) is defined as the lowest concentration of an antimicrobial that inhibits the growth of a microorganism after incubation. MIC, therefore, is crucial to be able to confirm the resistance of microorganisms to an antimicrobial agent as well as to monitor the activity of new antimicrobial agents [18]. Classically, there are both time-dependent and concentration-dependent antibiotics. Time-dependent antibiotics are considered adequate to treat an infection caused by a bacterium when, administered in doses considered therapeutic (i.e., non-toxic), they reach a plasma concentration at the site of infection at least four times higher than the MIC most of the time. Concentration-dependent antibiotics are those that are considered adequate to treat an infection when they reach a peak concentration 10–12 times above the MIC value. For the interpretation of susceptibility tests, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) defined the following categories since 2019 [19]: S: sensitive. Thus, a bacterium that is inhibited in vitro by a concentration of the antimicrobial is associated with a high probability of therapeutic success. I: (formerly intermediate) sensitive with increasing exposure. In this case, the microorganism is sensitive when exposure to the antibiotic is increased; therefore, there is a high probability of therapeutic success because exposure to the agent is increased by adjustment of the dosage regimen or by its concentration at the site of infection. A: resistant. The treatment of the microorganism studied for the given antibiotic has a high probability of therapeutic failure. MIC is not only used to determine the concentration of antimicrobial that the patient will receive but also the type of antimicrobial to be used, allowing for reduction in the opportunity for microbial resistance to specific antimicrobial agents to develop. This does not mean that it is necessary to choose antimicrobials that have a lower MIC value, as other factors, such as the pharmacokinetics of the antimicrobial and the concentration reached at the site of infection, play a role. However, a lower MIC value induces a higher probability of achieving the therapeutic target and the possibility of a lower development of toxicity and resistance mechanisms, especially mutational ones. It is important to note that MIC is defined for each microorganism with respect to each antibiotic; therefore, strictly speaking, the fact that one antibiotic has a lower MIC than another for the same microorganism does not have therapeutic implications. On the other hand, referring to the same antibiotic for a certain microorganism (e.g., an MIC of 1 μg/mL or 8 μg/mL), despite both possessing “sensitive” MIC values, will have therapeutic implications.4.3. Preventive Concentration of Mutants
Mutant preventive concentration (MPC) is the MIC of the least sensitive mutant among a heterogeneous bacterial population and represents the lowest antibiotic concentration that prevents or blocks the emergence of resistant mutants [20]. There is a window of concentrations that prevents the emergence of resistant mutants. The exposure of a bacterial population to the action of an antimicrobial usually produces a deleterious effect on it, inhibiting its growth or causing its death. This effect is not always obvious, either due to the presence of a previous resistance mechanism in it or due to the emergence of resistant mutants. During antimicrobial administration, selection of resistant mutants can occur dynamically over time. This is related to the dose of the antimicrobial and its frequency, its pharmacokinetics at the site of infection, and the time during which concentrations equal to or greater than the MPC are reached. Antimicrobials can be described as having a mutagenic effect and inducing mutations. MPC is not strictly a parameter of antimicrobial activity, which would be better determined by the value of the MIC and its correct interpretation; however, it could be used as such. Its application to the ability to restrict or decrease the selection of resistant mutants when antimicrobials are used with Pk/Pd criteria and, therefore, to the possibility of avoiding associated clinical failure would lead to the assumption that MPC is an activity parameter.4.4. The Importance of Rectal Exudate
The microbiological tests in critically ill patients highlight the role that rectal exudate can play as an effective and high-yield surveillance culture that can determine which microorganism is potentially causing a serious infection and how to act based on the results. The result of this test, available in 24–36 h, can be very useful, when the causative microorganism is not identified, to help one to decide what to designate as the definitive empiric antibiotic treatment. Most nosocomial infections are endogenous and originate in the mucosal microbiota. This endogenous infection occurs due to translocation of the predominant aerobic microorganisms in the intestinal mucosa, and the likelihood of its diagnosis depends on its density and colonized area. The significant or repeated presence of a GNB in the rectal exudate implies its overgrowth in the intestinal lumen, and the relationship between intestinal colonization and the risk of bacteremia, infection by carbapenemase-producing microorganisms, or candidemia, among others has been verified [21]. The composition of the gut microbiota can change within 72 h of the arrival of a new microorganism or the start of antibiotic treatment; thus, determining the microorganisms that make up that microbiota and their sensitivities provides us important indices concerning the possible causes of infections.5. Pharmacokinetics and Pharmacodynamics of Beta-Lactams
Given the scarcity of effective drugs against new strains of resistant drugs, much more attention has been paid to Pk/Pd, and Pk/Pd has been granted the importance it deserves when choosing antibiotics and how they are administered [22]. Although the expected clinical efficacy of this proposal has not been demonstrated, either due to an absence of effect, insufficient sizes in the comparative studies carried out, or even due to possible methodological errors, studies as influential as DALI that demonstrated insufficient exposure [23] and the influence of authorities and societies have made prolonged infusions of beta-lactams a standard in the ICU [24]. In this regard, it is interesting to comment on the results of a recent randomized controlled trial (RCT) with apparently contradictory results on this topic. Regarding meropenem, to date, the administration of a total daily fractionated dose was recommended, but in an extended infusion of 3–4 h. The RCT “MERCY Trial” has shown that in critically ill patients with sepsis or septic shock, there were no differences in mortality at 28 days or in the appearance of resistance between continuous infusion administration of the same dose of 3 g in 24 h and intermittent administration (1 g every 8 h, administered over 30–60 min). As indicated in the editorial accompanying the study, since infusions do not cause harm or entail an additional cost, it is difficult for the guidelines to discuss even the published meta-analyses that indicate a possible decrease in mortality [25]. Furthermore, in this sense, there has been interest in the administration of antibiotics through routes other than intravenous for the treatment of certain infections. This is the case for aerosolization for the prevention and treatment of respiratory infections related to intubation, usually associated with intravenous administration. However, the lack of sufficient RCTs [26], together with the need for specific devices for proper aerosolization, means that aerosolization remains a practice not recommended by the American Thoracic Society (ATS), the Centers for Disease Control and Prevention (CDC), or the European Society of Clinical and Infectious Diseases (ESCMID) [27][28][29].6. Allergy to Beta-Lactams
The label of allergy to beta-lactams is often self-imposed by the patients themselves, and, in a large number of cases, this label is applied due to the appearance of symptoms that do not even possess an underlying immune mechanism (diarrhea, headache, etc.). It is estimated that this label has been applied to 15% of the general population and is true in less than 10% of these cases (therefore, only in approximately 1% of the population) [30][31]. Strictly speaking, only immunological reactions mediated by IgE (type I of the Gell and Coombs classification) are considered true allergies. The presentation of these reactions is varied and, generally, appear in the first hour following administration of the drug. Although not all of these reactions are severe, the most severe forms (anaphylaxis and bronchoconstriction) are life threatening and contraindicate subsequent administration of the drug that causes them. The drug challenge or oral test is still the gold standard of diagnosis. However, it is notable that scientific societies generally consider screening through antecedents and clinical history to be insufficient [32][33], while many authors require its use in emergency situations and propose an approach that allows safe and immediate administration in severe cases where there are no plausible alternatives [34][35][36][37].7. What Should I Know about Old Antibiotics
An interesting study carried out by the ESCMID Study Group for Antibiotic Policies in several European countries, the USA, Canada, and Australia showed that drugs such as cloxacillin or aztreonam were only available in about half of these countries, that not all of them had colistin, and that only 5 out of a total of 38 possessed colistin for the intravenous formulation of fosfomycin [38]. Among these drugs demonstrating activity against GNB are fosfomycin, colistin, and cotrimoxazole. All of these have hardly any activity against anaerobes, and their activity against GPB is variable. Regarding fosfomycin, there seems to be consensus on its usefulness only as an alternative to nitrofurantoin or trimethoprim-sulfamethoxazole (TMP-SMX) in uncomplicated cystitis caused by GNB-producing extended-spectrum beta-lactamases (ESBLs) or carbapenemases. In renal parenchyma, it does not reach sufficient concentrations, so it is not recommended in cases of complicated urinary tract infection (UTI), bacteremia, or other serious infectious conditions with a non-urinary focus and should only be used when the causative germ is E. coli, since K. pneumoniae and other Gram-negative causes of the condition are frequent carriers of fosA hydrolase that inactivates the drug. Colistin, with a known nephrotoxic potential and a narrow therapeutic index, was formerly recommended in antibiotic therapy association schemes to treat carbapenemase-producing GNB or Pseudomonas aeruginosa MDR, but the availability of the new BL-BLI and the growing increase in resistance to colistin due to its use in recent years have contributed to this practice being advised against. Cotrimoxazole plays a key role in UTIs (complicated or not), both those caused by ESBL-producing enterobacteriaceae and those resistant to carbapenems. It loses its usefulness against Pseudomonas aeruginosa but recovers it against Stenotrophomonas maltophilia, against which it is one of the treatments of choice [39]. Tigecycline has broad-spectrum activity that makes it an attractive option for MDR infection [40]. However, it has been reported that it can lead to treatment failure in some contexts if used as monotherapy, as it is a bacteriostatic antibiotic [41]. Therefore, these alternatives should not generally be recommended for current empirical use nor, in the context of septic shock, in environments with a high proportion of MDR. TMP-SMX only seems to be recommended for infections that require a long treatment time (bacteremia and nosocomial pneumonia) and once the susceptibility of the germ to it has been demonstrated [42]. Rifampicin and clindamycin are only recommended as part of a combination regimen, in association with first-line treatment, in certain conditions such as osteomyelitis, necrotizing pneumonia, meningitis, and, especially, in the case of rifampicin, endocarditis on the prosthetic valve, due to the potential greater eradication effect of rifampicin on prosthetic material [43]. Finally, it is worth noting the potential role of quinupristin-dalfopristin in the alternative treatment of infections caused by E. faecium when this germ presents resistance to vancomycin, linezolid, and daptomycin [38].8. New Antibiotics
8.1. Ceftazidime/Avibactam
Ceftazidime/avibactam is a combination of an existing cephalosporin, ceftazidime (inhibitor of bacterial cell wall synthesis, which causes cell death), and a new inhibitor of non-ß-lactam ß-lactamase, avibactam (inactivator of certain beta-lactamases by covalent acylation of beta-lactamases and which does not, per se, possess antibacterial properties). The addition of avibactam restores the in vitro activity of ceftazidime against a significant number of beta-lactamases, including class A enzymes of the Ambler classification (ESBL and K. pneumoniae carbapenemases (KPCs)), class C enzymes (Amp C), and certain class D enzymes (OXA-48 carbapenemases). It does not exhibit activity in the presence of class B enzymes (metallo-ß-lactamases) [44]. Therefore, it has the spectrum of antipseudomonic actions of ceftazidime and is active against other GNB, in general, and against Enterobacterales, in particular. However, it does not show activity against Acinetobacter, Stenotrophomonas maltophilia, or anaerobes (thus, it is worth recalling that an anaerobicide antibiotic must be associated with intra-abdominal infection). The antibiotic is registered by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to treat complicated intra-abdominal infections, complicated urinary tract infections, hospital-acquired pneumonia, and pneumonia associated with mechanical ventilation [45].8.2. Ceftolozane/Tazobactam
Ceftolozane/tazobactam is a new antibiotic composed of a new cephalosporin associated with tazobactam, a known β-lactamase inhibitor. Ceftolozane/tazobactam is active against Pseudomonas aeruginosa MDR bacteria, including those resistant to carbapenems, and against ESBL-producing enterobacteriaceae. However, it is not active in the case of CRE. The drug is approved by the FDA and EMA for the treatment of intra-abdominal infections, complicated urinary tract infections, and hospital-acquired and ventilator-associated bacterial pneumonia [46].8.3. Cefiderocol
Cefiderocol is a catechol-type cephalosporin siderophore with very potent in vitro activity against drug-resistant CRE and non-fermenting GNB. It uses the siderophore–iron complex pathway to penetrate Gram-negative membranes through a mechanism that has been compared to the well-known Trojan horse. Once inside the bacterium, cefiderocol separates from iron and binds to penicillin-binding proteins to inhibit peptidoglycan synthesis [47]. This antibiotic appears to be more stable against the hydrolysis of many ß-lactamases and carbapenemases. Its spectrum of action—in principle, the broadest of the antibiotics marketed for GNB, including multidrug-resistant antibiotics and especially metallo-beta-lactamase producers—has led to high expectations for this drug [48].9. Cutting-Edge Approaches to New Antibiotics
9.1. Sparing Carbapenems
The concept of sparing carbapenem aims to save this type of antibiotic and judiciously use new drugs that can participate in this strategy, keeping in mind the appropriate infection control and prevention measures within stewardship strategies [49]. Carbapenems are excellent antibiotics and a powerful weapon against drug-resistant Gram-negative bacteria because of their broad spectrum, their bactericidal effect, and their ability to circumvent many resistances. Their excessive use, in the absence of safe alternatives to date, especially in empirical treatment when there is a risk of MDR, has generated a problem of resistance to carbapenems, especially carbapenemases [50]. Intestinal colonization is the main reservoir of carbapenem-resistant GNB (CRE) among critically ill patients, and intestinal translocation is associated with an increased risk of developing CRE in successive infections. Not surprisingly, overuse of carbapenems is one of the critical determinants for CRE acquisition and is associated with a higher rate of superinfection compared to non-carbapenem treatments. With carbapenem treatment, there is an increased risk of developing resistance due to the pressure carbapenems exert on sensitive strains, providing resistant strains the opportunity to overcome them [51].9.2. Role of New Antibiotics in the Empirical Treatment of Nosocomial Sepsis and Septic Shock
Undoubtedly, the most difficult and controversial issues in the treatment of sepsis include determining a personalized empirical antibiotic treatment and defining the role played by new antibiotics in this empirical regimen [52]. Although targeted therapy against GNB is not without controversy either, today scholars have several guidelines for targeted treatment against MDR germs (producers of ESBL and/or carbapenems, difficult-to-treat Pseudomonas, or other problematic germs such as Acinetobacter or Stenotrophomonas). These guidelines are very balanced and indicative and have a very similar background, often based on up-to-date expert opinion, due to the rapid emergence of new drugs and published evidence, or using a GRADE methodology [53][54][55]. Beyond differences related to the positioning of particular drugs due to contradictory trials and the role played in previous years by old antibiotics used for MDRs, antibiotics are very clearly positioned, particularly those aforementioned drugs that have been the subject of numerous publications and consistent clinical experience, such as ceftazidime/avibactam or ceftolozane/tazobactam.10. Recommendations for Empiric Antibiotic Treatment of Patients in Septic Shock
10.1. Preliminary Considerations
The antibiotic treatment of critically ill patients is like a children’s puzzle composed of several pieces that are combined to promote clinical cure and the prevention of the development of resistance: the antibiotic and/or antifungal and its properties, the site of infection, the pathogen responsible and its susceptibility to treatment (MIC), and the patient’s pathophysiology (Figure 1).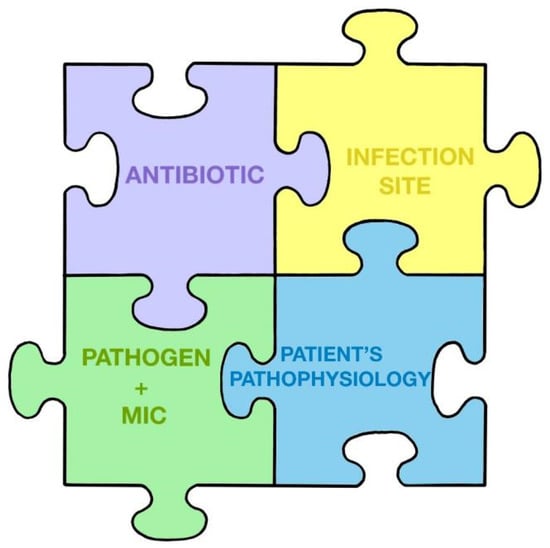 Figure 1. The puzzle of antibiotic treatment according to Pea and Viale.The empirical guidelines will be greatly influenced by the suspicion of the focus of infection, since this can vary with, e.g., pneumonia or an intra-abdominal infection.In relation to the importance of reducing the bacterial load, it is interesting to know how the bacterial load affects the effect of antibiotics, recalling that when the bacterial load is very high, ceftazidime/avibactam may be the least affected in this context [56][57]. Finally, an important process, as Bassetti and Montravers point out, is to assess the local prevalence of MDR infections [58]. If the percentage of resistance to carbapenems is greater than 20%, an empiric antibiotic treatment strategy using new antibiotics would make sense, even in cases of sepsis and not only in septic shock. This should be applied, again, depending on the focus of infection.Therefore, decision making regarding the initial empiric antibiotic treatment of patients in septic shock should consider the following premises:
Figure 1. The puzzle of antibiotic treatment according to Pea and Viale.The empirical guidelines will be greatly influenced by the suspicion of the focus of infection, since this can vary with, e.g., pneumonia or an intra-abdominal infection.In relation to the importance of reducing the bacterial load, it is interesting to know how the bacterial load affects the effect of antibiotics, recalling that when the bacterial load is very high, ceftazidime/avibactam may be the least affected in this context [56][57]. Finally, an important process, as Bassetti and Montravers point out, is to assess the local prevalence of MDR infections [58]. If the percentage of resistance to carbapenems is greater than 20%, an empiric antibiotic treatment strategy using new antibiotics would make sense, even in cases of sepsis and not only in septic shock. This should be applied, again, depending on the focus of infection.Therefore, decision making regarding the initial empiric antibiotic treatment of patients in septic shock should consider the following premises:-
The bacterial load at the focus of infection will usually be high. The higher the bacterial load in the focus of infection, the higher the concentration of antibiotic needed to inhibit the growth of the microorganism and the greater the probability of selecting resistant mutants if the administered dose is not sufficient. The antibiotic exposure required to suppress the emergence of resistance should be maintained above the mutant selection window. Control of the outbreak via surgery or drainage is essential to decrease the bacterial load when possible.
-
As we do not know the microorganisms causing the infection or their patterns of antibiotic susceptibility, we can be guided by the suspected outbreak or guided by surveillance cultures, especially rectal exudate within the previous 48–72 h [52].
-
The local ecology of MDR bacteria influences the decision. A high percentage of resistance to carbapenems makes it necessary to consider the use of new antibiotics [59].
-
Lack of training;
-
Lack of correct assessment of severity and delay in recognition of sepsis;
-
Increased work;
- Empiric treatment should be active against all potentially involved microorganisms and, whenever possible, should contain a β-lactam antibiotic for its efficacy, spectrum, and bactericidal effect. Among the β-lactam antibiotics currently available, the most recommended for any outbreak, due to their antibacterial spectrum and probability of reaching the optimal target of Pk/Pd against MDR GNB, are ceftazidime/avibactam and meropenem. Depending on the focus of infection and colonization, personalization may be necessary and alternatives may exist.
- Transfer of the patient (to the operating room, to tests, etc.);
- The pharmacokinetics of antibiotic administration in sepsis will be drastically influenced by the inherent characteristics of the critically ill patient
- [
- ]
- .
- Possibility of atypical manifestations, without fever or confusion;
- A patient in shock is at risk of irreversible damage, so it is urgent to reduce the bacterial load and control the immune response, while offering organ support.
- Initial empirical therapy for patients in septic shock with pneumonia should be with two antibiotics and be based on risk factors for MDR pathogens discussed above, with an initial approach based on broad-spectrum therapy, followed by a reduction if MDR pathogens are ruled out in cultures
- [
- ]
- .
-
Complicated intra-abdominal infection is usually polymicrobial, often with the intervention of GNB, anaerobes, and enterococci. Initial empiric therapy of patients in septic shock in this setting should include beta-lactam with a beta-lactamase inhibitor or carbapenem as well as coverage for anaerobes if beta-lactam does not cover them, coverage for enterococcus if the infection is nosocomial, and, in some patients with risk factors, additional initial coverage should be added for Candida species other than C. albicans. In this situation, it is key to control the outbreak, obtain quality samples, and process them quickly [62].
-
Daptomycin, linezolid, tedizolid, or vancomycin can be used against GPB. The choice depends on the location of the infection, renal function, and the need to use other nephrotoxic drugs simultaneously. It should be recalled that daptomycin does not show activity in the pulmonary focus [63].
-
Fungal infection accounts for 5% of sepsis. It is most commonly due to Candida spp. and can be predicted by specific scores such as the Candida Score as well as by epidemiological data, microbiological data, and biomarkers. Risk factors overlap with those of other causes of sepsis in the ICU. Preventive and empirical therapy is often necessary, and echinocandins are preferred for Candida, but some strains are becoming resistant so, in some scenarios, such as intra-abdominal infection or cases that are difficult to treat, liposomal amphotericin B may be a suitable alternative [64][65].
-
Appropriate cultures should always be obtained, depending on the focus of infection (blood cultures, rectal exudate, or other surveillance cultures, depending on the context), before the start or shortly after the initial empiric antibiotic treatment. The alarm, e.g., the sepsis code, should be triggered to ensure that samples are processed with rapid microbiological identification systems and, if possible, the microbiologist on duty should be alerted and interacted with [66
- ]
- .
- After the first 24–48 h, the treatment should be re-evaluated in the context of a decrease in bacterial load and clinical improvement. Rapid diagnostic techniques will have allowed us to assess the absence or presence of ESBL-producing enterobacteriaceae or carbapenemases, resistant
- P. aeruginosa
-
The presence of hypotension in a patient with suspected infection should be considered septic shock in the absence of an alternative explanation;
- Delays due to other diagnostic tests and slow collection of microbiological samples;
- , MRSA, as well as a low value of β-D-glucan or its positivity, which will allow us to narrow the antibiotic spectrum or suggest a need to change treatment
- [
- Failure to transfer or transport the patient before antibiotic treatment has been administered;
- ]
- .
- Short-term treatments should be attempted. Biomarkers such as procalcitonin (PCT) can help us in the application of short antibiotic treatment regimens
- [
- ]
- .
10.2. Individualization of Antibiotic Treatment in Critically Ill Patients
Although the main challenges, in this context of the high prevalence of resistant strains, are to avoid delaying diagnosis through the rapid identification of microorganisms and their sensitivity patterns, adequate treatment is complicated by the peculiarities of the most severely ill patients who present alterations in their extracellular volume, renal function, and immunity, among other symptoms. In this way, the severity of their condition not only produces a higher mortality rate per se, but also affects the clinical response to antimicrobial treatment.
10.3. Early Antibiotic Treatment in Patients in Septic Shock
The current standard of antibiotic treatment for critically ill patients is based on early treatment and involves the administration of antibiotics in less than an hour in the most severely ill patients or those presenting with septic shock [- MDR germ infections and not recognizing MDR risk factors;
- Waiting to carry out blood cultures before providing the antibiotic;
10.7. Tools for Making Sound Decisions
Among the tools available to improve the care of critically ill, infected patients are cognitive aids, the paradigmatic example of which are published antibiotic treatment guidelines. The Antimicrobial Therapeutics Guide (Mensa) (Mensa Guide—Apps on Google Play) is the most widely used guide in Spain, with more than 20 editions. It is very intuitive, complete, and easy to use.-
Annual update;
-
Provides local epidemiology data;
-
It is a guide made in the USA, available in English;
-
Epidemiology contains data from the United States.
-
Characteristics of antimicrobials;
-
Dosing of antimicrobials in special situations;
-
Treatment of infections caused by specific microorganisms.
- Very importantly, failing to recognize that providing an inappropriate antibiotic is like giving none or even worse. Appropriate antibiotics should not be delayed by inappropriate administration;
- Not specifying the order in which antibiotics are administered and what the broad-spectrum “key” antibiotic is;
-
Needing to obtain authorization for the prescription, either from another specialist, the head of the infectious diseases department, or the pharmacy, before starting treatment.
- If an antibiotic is requested in these situations, it must be ensured that it is administered immediately (it has been reported that the delay from the time the treatment is written to the time it is administered can be up to 3 h on average);
-
Combination regimens should be administered with the key antibiotic (the broadest spectrum) provided first;
-
Promote education, training, and teamwork to reduce administration time.
10.4. The Importance of Focus Control in Surgical Units
The management of infection in the ICUs of surgical patients presents its own challenges since, as in other settings, diagnosis is often problematic and multidrug resistance and pharmacokinetic changes present challenges. The need to control the focus of infection, either by surgical and/or percutaneous procedure, adds complexity to the case [74]. Restoring anatomy and function is equally important and can be carried out in the same operation, although sometimes the decision to perform it in this way is difficult. As a rule of thumb, measures to control the focus of infection should not be delayed except in those situations where demarcation of non-viable tissue is preferable, such as in infected necrotic pancreatitis or in situations where the source of control is difficult to access. Nowadays, there is a renewed interest in so-called damage control surgery in intra-abdominal infection, which involves the initial control of the infection through an abbreviated laparotomy and the use of an initial technique that leaves the laparotomy open in a way that minimizes the risk of damage, facilitates the cleaning of the peritoneal cavity, and decreases the risk of abdominal hyperpressure and compartment syndrome [75]. The SSC guidelines recommend identifying an anatomical source of infection that may require control of the outbreak and resolving it as soon as possible from a logistical and medical point of view. The approach should be individualized: imaging tests are very important and should not be limited to the abdominal cavity [76]. There is no point in delaying such control unless there are significant metabolic or coagulation alterations or the patient is very unstable hemodynamically. The appropriate timing to control the outbreak is a matter of debate, and one should adopt a multidisciplinary approach guided by the severity of the infection, the speed of deterioration of the patient, the presumed source of infection, and the pathophysiological state of the patient. There are pieces of data indicating that in the most severe infections, controlling the focus of infection produces better results when it is carried out between 2 and 6 h after diagnosis [77]. Surgical infections, specifically abdominal infections, are the most complex among nosocomial infections: they involve longer average stays, more patients in shock, and a greater occurrence of renal failure with higher mortality. Their microbiological pattern is usually polymicrobial with a non-negligible presence of fungi, difficulties in establishing the pathogenic role of each of them and their contribution to the patient’s overall condition, and a growing presence of multidrug-resistant organisms, especially carbapenemase-producing organisms [62]. Inflammation associated with surgical trauma means that the traditional criteria of systemic inflammatory response are present on a regular basis and are, therefore, of little use in the diagnosis of an infectious process, as are the analytical markers of infection. Similarly, there is little use in measurements regarding leukocytosis, PCT value (although an increase in the latter, doubling its initial value in the first 24 h after surgery, can help to warn of a failure to control the focus of infection), or other signs, such as hypotension or oliguria, which may be related to postoperative complications of non-infectious origin [78]. All of this complicates the decision-making process regarding whether or not to start antibiotic treatment and with which drugs. In addition, if the patient’s serious situation is prolonged, de-escalation will also be difficult. If excellent control is assumed, antibiotic treatment of abdominal infection should be limited to 3–5 days. This seems to be demonstrated in nosocomial infections, even in patients with APACHE > 10, with no increase in relaparotomies or increases in mortality [79].10.5. Optimization of Antibiotic Treatment According to Kumar’s Hypothesis
After witnessing a decrease in mortality rates from infectious causes with the advent of penicillin, the absolute mortality figures remain constant today despite the emergence of new antimicrobials. This is due to a higher prevalence of resistant microorganisms, a higher incidence of sepsis in older populations, and the existence of more aggressive microorganisms [80]. Other supportive measures and treatments unrelated to antibiotics have been questioned following dozens of studies. Kumar’s hypothesis is that tested therapies have failed because the disease process has not been properly interpreted [81]. The alternative pathophysiological model proposed by Kumar suggests the substantial implication of bacterial load as the main driver of organ dysfunction and proposes that bacterial load should, therefore, guide the antibiotic regimen in sepsis. The key to improving outcomes may then be to reduce this burden as soon as possible based on better administration of available antibiotics and other strategies that aim at this end.10.6. Duration of Antibiotic Treatment within Stewardship Programs
11. Executive Summary for the Individualization of Antibiotic Treatment in Critically Ill Patients
Different studies confirm the relationship between delay in initiating appropriate antibiotic treatment and mortality [70][88]. In GNB infections, inappropriate antibiotic treatment increased the risk of mortality by almost four times [89]. In addition, the need to quickly determine correct antibiotic treatment is extremely important in patients with sepsis or septic shock for whom, even with adequate antibiotic treatment, mortality can reach between 27% and 40% in patients with multiple comorbidities and a functional reserve limited by their frailty and in patients with some degree of immunosuppression [90]. Knowledge of local epidemiology is essential to initiate appropriate empirical treatment. Knowing the total rate of carbapenem resistance of GNB with epidemiological importance in each service and hospital can be used as an indicator of risk for the patient of the presence of colonization or infection by these microorganisms. Most hospital-acquired infections are infections that originate from the endogenous microbiota of the mucosal surfaces via translocation or invasion of the predominant microorganisms, depending on the density of the bacterial population. Therefore, knowing the colonizing flora and their pattern of antimicrobial susceptibility may be important in the choice of initial empirical treatment. For all these reasons, it would seem reasonable to perform surveillance cultures upon admission to the ICU and subsequently one to two times a week, although changes in the composition of the microbiota before the sepsis episode occurs cannot be ruled out. It is also important to assess the site of infection. In patients with risk factors for carbapenem-resistant GNB, the use of new antibiotics should be considered when the clinical efficacy of possible alternatives is expected to be suboptimal, as is the case with polymyxins and/or aminoglycosides in patients with pneumonia [91][92].
References
- Cortegiani, A.; Antonelli, M.; Falcone, M.; Giarratano, A.; Girardis, M.; Leone, M.; Pea, F.; Stefani, S.; Vaggi, B.; Viale, P. Rationale and clinical application of antimicrobial stewardship principles in the intensive care unit: A multidisciplinary statement. J. Anesth. Analg. Crit. Care 2023, 3, 11.
- Liebchen, U.; Briegel, J.; Brinkmann, A.; Frey, O.; Wicha, S.G. Individualised dosing of antibiotics in ICU patients: Timing, target and model selection matter. Intensive Care Med. 2023, 49, 475–476.
- Yoo, J.H. The Infinity War: How to Cope with Carbapenem-resistant Enterobacteriaceae. J. Korean Med. Sci. 2018, 33, e255.
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387.
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241.
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19.
- De Waele, J.J.; Boelens, J.; Leroux-Roels, I. Multidrug-resistant bacteria in ICU: Fact or myth. Curr. Opin. Anaesthesiol. 2020, 33, 156–161.
- Bassetti, M.; Righi, E.; Vena, A.; Graziano, E.; Russo, A.; Peghin, M. Risk stratification and treatment of ICU-acquired pneumonia caused by multidrug- resistant/extensively drug-resistant/pandrug-resistant bacteria. Curr. Opin. Crit. Care 2018, 24, 385–393.
- The Abdominal Sepsis Study (AbSeS) Group on behalf of the Trials Group of the European Society of Intensive Care Medicine; Blot, S.; Antonelli, M.; Arvaniti, K.; Blot, K.; Creagh-Brown, B.; de Lange, D.; De Waele, J.; Deschepper, M.; Dikmen, Y.; et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019, 45, 1703–1717.
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12.
- Wunderink, R.G.; Srinivasan, A.; Barie, P.S.; Chastre, J.; Dela Cruz, C.S.; Douglas, I.S.; Ecklund, M.; Evans, S.E.; Evans, S.R.; Gerlach, A.T.; et al. Antibiotic Stewardship in the Intensive Care Unit. An Official American Thoracic Society Workshop Report in Collaboration with the AACN, CHEST, CDC, and SCCM. Ann. Am. Thorac. Soc. 2020, 17, 531–540.
- Mensa, J.; Barberán, J.; Ferrer, R.; Borges, M.; Rascado, P.; Maseda, E.; Oliver, A.; Marco, F.; Adalia, R.; Aguilar, G.; et al. Recommendations for antibiotic selection for severe nosocomial infections. Rev. Esp. Quimioter. 2021, 34, 511–524.
- Peri, A.M.; Stewart, A.; Hume, A.; Irwin, A.; Harris, P.N.A. New Microbiological Techniques for the Diagnosis of Bacterial Infections and Sepsis in ICU Including Point of Care. Curr. Infect. Dis. Rep. 2021, 23, 12.
- Tamma, P.D.; Miller, M.A.; Cosgrove, S.E. Rethinking How Antibiotics Are Prescribed: Incorporating the 4 Moments of Antibiotic Decision Making Into Clinical Practice. JAMA 2019, 321, 139–140.
- Gupta, E.; Saxena, J.; Kumar, S.; Sharma, U.; Rastogi, S.; Srivastava, V.K.; Kaushik, S.; Jyoti, A. Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods. Diagnostics 2023, 13, 277.
- Gerace, E.; Mancuso, G.; Midiri, A.; Poidomani, S.; Zummo, S.; Biondo, C. Recent Advances in the Use of Molecular Methods for the Diagnosis of Bacterial Infections. Pathogens 2022, 11, 663.
- Hansen, G.T. Point-of-Care Testing in Microbiology: A Mechanism for Improving Patient Outcomes. Clin. Chem. 2020, 66, 124–137.
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165.
- Nabal Díaz, S.G.; Algara Robles, O.; García-Lechuz Moya, J.M. New definitions of susceptibility categories EUCAST 2019: Clinic application. Rev. Esp. Quim. 2022, 35 (Suppl. S3), 84–88.
- Drlica, K.; Zhao, X. Mutant Selection Window Hypothesis Updated. Clin. Infect. Dis. 2007, 44, 681–688.
- Schlebusch, S.; Graham, R.M.A.; Jennison, A.V.; Lassig-Smith, M.M.; Harris, P.N.A.; Lipman, J.; Ó Cuív, P.; Paterson, D.L. Standard rectal swabs as a surrogate sample for gut microbiome monitoring in intensive care. BMC Microbiol. 2022, 22, 99.
- Roberts, J.A.; Taccone, F.S.; Lipman, J. Understanding PK/PD. Intensive Care Med. 2016, 42, 1797–1800.
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083.
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 104.
- Hong, L.T.; Downes, K.J.; FakhriRavari, A.; Abdul-Mutakabbir, J.C.; Kuti, J.L.; Jorgensen, S.; Young, D.C.; Alshaer, M.H.; Bassetti, M.; Bonomo, R.A.; et al. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists: An executive summary. Pharmacotherapy 2023, 43, 736–739.
- Tang, R.; Luo, R.; Wu, B.; Wang, F.; Song, H.; Chen, X. Effectiveness and safety of adjunctive inhaled antibiotics for ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. J. Crit. Care 2021, 65, 133–139.
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111.
- Tablan, O.C.; Anderson, L.J.; Besser, R.; Bridges, C.; Hajjeh, R.; CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2004, 53, 1–179.
- Rello, J.; Solé-Lleonart, C.; Rouby, J.J.; Chastre, J.; Blot, S.; Poulakou, G.; Luyt, C.-E.; Riera, J.; Palmer, L.B.; Pereira, J.M.; et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2017, 23, 629–639.
- Shenoy, E.S.; Macy, E.; Rowe, T.; Blumenthal, K.G. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019, 321, 188–199.
- Stone, C.A.; Trubiano, J.; Coleman, D.T.; Rukasin, C.R.F.; Phillips, E.J. The challenge of de-labeling penicillin allergy. Allergy 2020, 75, 273–288.
- Alvarez-Cuesta, E.; Madrigal-Burgaleta, R.; Broyles, A.D.; Cuesta-Herranz, J.; Guzman-Melendez, M.A.; Maciag, M.C.; Phillips, E.J.; Trubiano, J.A.; Wong , J.T.; Ansotegui, I. Standards for practical intravenous rapid drug desensitization & delabeling: A WAO committee statement. World Allergy Organ. J. 2022, 15, 100640.
- Savic, L.; Ardern-Jones, M.; Avery, A.; Cook, T.; Denman, S.; Farooque, S.; Garcez, T.; Gold, R.; Jay, N.; Krishna, M.T.; et al. BSACI guideline for the set-up of penicillin allergy de-labelling services by non-allergists working in a hospital setting. Clin. Exp. Allergy 2022, 52, 1135–1141.
- Trubiano, J.A.; Vogrin, S.; Chua, K.Y.L.; Bourke, J.; Yun, J.; Douglas, A.; Stone, A.C.; Yu, R.; Lauren, G.; Holmes, N.E.; et al. Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA Intern. Med. 2020, 180, 745–752.
- Courtemanche, J.; Baril, L.; Clément, A.; Bédard, M.A.; Plourde, M.; Émond, M. Is it possible to identify patients at low risk of having a true penicillin allergy? Can. J. Emerg. Med. 2022, 24, 366–368.
- Trubiano, J.A. A Risk-Based Approach to Penicillin Allergy. Immunol. Allergy Clin. North Am. 2022, 42, 375–389.
- Holmes, M.D.; Vo, N.; Rafeq, R.; Byrne, D.; King, M. Administration of β-lactam antibiotics to patients with reported penicillin allergy in the emergency department. Am. J. Emerg. Med. 2023, 68, 119–123.
- Pulcini, C.; Bush, K.; Craig, W.A.; Frimodt-Møller, N.; Grayson, M.L.; Mouton, J.W.; Turnidge, J.; Harbarth, S.; Gyssens, I.C.; ESCMID Study Group for Antibiotic Policies. Forgotten antibiotics: An inventory in Europe, the United States, Canada, and Australia. Clin. Infect. Dis. 2012, 54, 268–274.
- Brown, G.R. Cotrimoxazole—Optimal dosing in the critically ill. Ann. Intensive Care 2014, 4, 13.
- Yaghoubi, S.; Zekiy, A.O.; Krutova, M.; Gholami, M.; Kouhsari, E.; Sholeh, M.; Ghafouri, Z.; Maleki, F. Tigecycline antibacterial activity, clinical effectiveness, and mechanisms and epidemiology of resistance: Narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1003–1022.
- De Pascale, G.; Lisi, L.; Ciotti, G.M.P.; Vallecoccia, M.S.; Cutuli, S.L.; Cascarano, L.; Gelormini, C.; Bello, G.; Montini, L.; Carelli, S.; et al. Pharmacokinetics of high-dose tigecycline in critically ill patients with severe infections. Ann. Intensive Care 2020, 10, 94.
- Brown, N.M.; Goodman, A.L.; Horner, C.; Jenkins, A.; Brown, E.M. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC Antimicrob. Resist. 2021, 3, dlaa114.
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128.
- Shirley, M. Ceftazidime-Avibactam: A Review in the Treatment of Serious Gram-Negative Bacterial Infections. Drugs 2018, 78, 675–692.
- Matesanz, M.; Mensa, J. Ceftazidime-Avibactam. Rev. Esp. Quimioter. 2021, 34 (Suppl. S1), 38–40.
- Wang, L.T.; Lin, W.T.; Lai, C.C.; Wang, Y.H.; Chen, C.H.; Chang, Y.T.; Chen, C.-H.; Wang, C.-Y. The safety of ceftolozane-tazobactam for the treatment of acute bacterial infections: A systemic review and meta-analysis. Ther. Adv. Drug Saf. 2021, 12, 20420986211027096.
- Sansone, P.; Giaccari, L.G.; Coppolino, F.; Aurilio, C.; Barbarisi, A.; Passavanti, M.B.; Pota, V.; Pace, M.C. Cefiderocol for Carbapenem-Resistant Bacteria: Handle with Care! A Review of the Real-World Evidence. Antibiotics 2022, 11, 904.
- Maseda, E.; Suárez de la Rica, A. The role of cefiderocol in clinical practice. Rev. Esp. Quimioter. 2022, 35 (Suppl. S2), 39–44.
- Corcione, S.; Lupia, T.; Maraolo, A.E.; Mornese Pinna, S.; Gentile, I.; De Rosa, F.G. Carbapenem-sparing strategy: Carbapenemase, treatment, and stewardship. Curr. Opin. Infect. Dis. 2019, 32, 663–673.
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell Infect. Microbiol. 2022, 12, 823684.
- Arulkumaran, N.; Routledge, M.; Schlebusch, S.; Lipman, J.; Conway Morris, A. Antimicrobial-associated harm in critical care: A narrative review. Intensive Care Med. 2020, 46, 225–235.
- Strich, J.R.; Heil, E.L.; Masur, H. Considerations for Empiric Antimicrobial Therapy in Sepsis and Septic Shock in an Era of Antimicrobial Resistance. J. Infect. Dis. 2020, 222 (Suppl. S2), S119–S131.
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, ciad428.
- Pintado, V.; Ruiz-Garbajosa, P.; Aguilera-Alonso, D.; Baquero-Artigao, F.; Bou, G.; Cantón, R.; Grau, S.; Gutiérrez-Gutiérrez, B.; Larrosa, N.; Machuca, I.; et al. Executive summary of the consensus document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) on the diagnosis and antimicrobial treatment of infections due to carbapenem-resistant Gram-negative bacteria. Enferm. Infecc. Microbiol. Clin. 2023, 41, 360–370.
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547.
- Kumar, A. An alternate pathophysiologic paradigm of sepsis and septic shock: Implications for optimizing antimicrobial therapy. Virulence 2014, 5, 80–97.
- Danjean, M.; Hobson, C.A.; Gits-Muselli, M.; Courroux, C.; Monjault, A.; Bonacorsi, S.; Birgy, A. Evaluation of the inoculum effect of new antibiotics against carbapenem-resistant enterobacterales. Clin. Microbiol. Infect. 2022, 28, 1503.e1–1503.e3.
- Montravers, P.; Bassetti, M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr. Opin. Infect. Dis. 2018, 31, 587–593.
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76 (Suppl. S4), iv23–iv37.
- Heffernan, A.J.; Mohd Sazlly Lim, S.; Lipman, J.; Roberts, J.A. A personalised approach to antibiotic pharmacokinetics and pharmacodynamics in critically ill patients. Anaesth. Crit. Care Pain. Med. 2021, 40, 100970.
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383.
- Di Franco, S.; Alfieri, A.; Fiore, M.; Fittipaldi, C.; Pota, V.; Coppolino, F.; Sansone, P.; Pace, M.C.; Passavanti, M.B. A Literature Overview of Secondary Peritonitis Due to Carbapenem-Resistant Enterobacterales (CRE) in Intensive Care Unit (ICU) Patients. Antibiotics 2022, 11, 1347.
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11.
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355.
- Keane, S.; Geoghegan, P.; Povoa, P.; Nseir, S.; Rodriguez, A.; Martin-Loeches, I. Systematic review on the first line treatment of amphotericin B in critically ill adults with candidemia or invasive candidiasis. Expert Rev. Anti Infect. Ther. 2018, 16, 839–847.
- Princess, I.; Vadala, R. Clinical Microbiology in the Intensive Care Unit: Time for Intensivists to Rejuvenate this Lost Art. Indian J. Crit. Care Med. 2021, 25, 566–574.
- Burillo, A.; Bouza, E. Faster infection diagnostics for intensive care unit (ICU) patients. Expert Rev. Mol. Diagn. 2022, 22, 347–360.
- Méndez Hernández, R.; Ramasco Rueda, F. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J. Pers. Med. 2023, 13, 333.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, N.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247.
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589.
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755.
- Peltan, I.D.; Mitchell, K.H.; Rudd, K.E.; Mann, B.A.; Carlbom, D.J.; Hough, C.L.; Rea, T.D.; Brown, S.M. Physician Variation in Time to Antimicrobial Treatment for Septic Patients Presenting to the Emergency Department. Crit. Care Med. 2017, 45, 1011–1018.
- Roberts, R.J.; Alhammad, A.M.; Crossley, L.; Anketell, E.; Wood, L.; Schumaker, G.; Garpestad, E.; Devlin, J.W. A survey of critical care nurses’ practices and perceptions surrounding early intravenous antibiotic initiation during septic shock. Intensive Crit. Care Nurs. 2017, 41, 90–97.
- Montravers, P.; Blot, S.; Dimopoulos, G.; Eckmann, C.; Eggimann, P.; Guirao, X.; Paiva, J.A.; Sganga, G.; De Waele, J. Therapeutic management of peritonitis: A comprehensive guide for intensivists. Intensive Care Med. 2016, 42, 1234–1247.
- Ordoñez, C.A.; Caicedo, Y.; Parra, M.W.; Rodríguez-Holguín, F.; Serna, J.J.; Salcedo, A.; Franco, M.J.; Toro, L.E.; Pino, L.F.; Guzmán-Rodríguez, M.; et al. Evolution of damage control surgery in non-traumatic abdominal pathology: A light in the darkness. Colomb. Med. 2021, 52, e4194809.
- De Waele, J.J.; Girardis, M.; Martin-Loeches, I. Source control in the management of sepsis and septic shock. Intensive Care Med. 2022, 48, 1799–1802.
- De Pascale, G.; Antonelli, M.; Deschepper, M.; Arvaniti, K.; Blot, K.; Brown, B.C.; de Lange, D.; De Waele, J.; Dikmen, Y.; Dimopoulos, G.; et al. Poor timing and failure of source control are risk factors for mortality in critically ill patients with secondary peritonitis. Intensive Care Med. 2022, 48, 1593–1606.
- Maseda, E.; Suarez-de-la-Rica, A.; Anillo, V.; Tamayo, E.; García-Bernedo, C.A.; Ramasco, F.; Villagran, M.-J.; Maggi, G.; Gimenez, M.-J.; Aguilar, L.; et al. Procalcitonin-guided therapy may reduce length of antibiotic treatment in intensive care unit patients with secondary peritonitis: A multicenter retrospective study. J. Crit. Care 2015, 30, 537–542.
- For the DURAPOP Trial Group; Montravers, P.; Tubach, F.; Lescot, T.; Veber, B.; Esposito-Farèse, M.; Seguin, P.; Paugam, C.; Lepape, A.; Meistelman, C.; et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: The DURAPOP randomised clinical trial. Intensive Care Med. 2018, 44, 300–310.
- Prest, J.; Nguyen, T.; Rajah, T.; Prest, A.B.; Sathananthan, M.; Jeganathan, N. Sepsis-Related Mortality Rates and Trends Based on Site of Infection. Crit. Care Explor. 2022, 4, e0775.
- Cavaillon, J.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128.
- Baggs, J.; Jernigan, J.A.; Halpin, A.L.; Epstein, L.; Hatfield, K.M.; McDonald, L.C. Risk of Subsequent Sepsis within 90 Days after a Hospital Stay by Type of Antibiotic Exposure. Clin. Infect. Dis. 2018, 66, 1004–1012.
- Teshome, B.F.; Vouri, S.M.; Hampton, N.; Kollef, M.H.; Micek, S.T. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy 2019, 39, 261–270.
- Raman, K.; Nailor, M.D.; Nicolau, D.P.; Aslanzadeh, J.; Nadeau, M.; Kuti, J.L. Early Antibiotic Discontinuation in Patients with Clinically Suspected Ventilator-Associated Pneumonia and Negative Quantitative Bronchoscopy Cultures. Crit. Care Med. 2013, 41, 1656–1663.
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518.
- Rau, B.; Krüger, C.M.; Schilling, M.K. Procalcitonin: Improved biochemical severity stratification and postoperative monitoring in severe abdominal inflammation and sepsis. Langenbeck’s Arch. Surg. 2004, 389, 134–144.
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474.
- Kollef, M.H.; Shorr, A.F.; Bassetti, M.; Timsit, J.F.; Micek, S.T.; Michelson, A.P.; Garnacho-Montero, J. Timing of antibiotic therapy in the ICU. Crit. Care 2021, 25, 360.
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596.
- Huang, C.T.; Tsai, Y.J.; Tsai, P.R.; Yu, C.J.; Ko, W.J. Severe Sepsis and Septic Shock: Timing of Septic Shock Onset Matters. Shock 2016, 45, 518–524.
- Panidis, D.; Markantonis, S.L.; Boutzouka, E.; Karatzas, S.; Baltopoulos, G. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 2005, 128, 545–552.
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39.
