You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Moritz Petzold and Version 2 by Wendy Huang.
In the quest for sustainable energy solutions and environmental protection, the management of end-of-life (EoL) batteries has emerged as a critical issue. Batteries, especially lithium-ion batteries (LIBs), power a wide range of devices and are central to modern life. As society’s reliance on batteries grows, there is an urgent need for sustainable battery recycling methods that can efficiently recover valuable materials, minimize environmental impact, and support the circular economy.
- recycling
- electrode
- electrolyte
- separator
- spent batteries
- lithium-ion batteries (LIBs)
1. Introduction
The circular economy of LIBs consists of different phases, starting with the extraction of raw materials, through production and the use phase for disposal, reprocessing, recycling and the subsequent substitution of primary raw materials. In particular, the phases from disposal and collection to metallurgical processing into secondary raw materials play a central role in the recycling of the LIBs. In general, the end-of-life processes for LIBs contain most or all of the following process steps:
The following sections present the current state-of-the-art in sorting, processing and recycling LIBs. The dismantling and discharging steps occur mainly in the recycling of LIBs from electric mobility [6][28]. Depending on the recycling route, thermal deactivation of LIB cells is also required [9][31].
2. Basics of Lithium-Ion Batteries
LIB cells consist of two electrodes (anode and cathode), an electrolyte and a separator. The operation of an LIB cell is based on the principle of reversible lithium-ion intercalation and de-intercalation during charging and discharging [17][39]. Between the electrodes is an ion-conducting electrolyte containing a dissociated lithium-conducting salt, commonly used is LiPF6. Other components of the electrolyte are organic carbonates, such as diethyl carbonate, dimethyl carbonate and others [17][39]. A porous plastic film separates the two electrodes from each other and acts as a membrane for lithium ions [18][20]. The cathode of commercial LIB cells usually consists of a compound that can accept lithium ions, such as transition metal oxides or polyanion compounds [19][40]. The most commonly used transition metal oxides are the following [20][21][22][23][24][25][26][21,41,42,43,44,45,46]:
-
Lithium cobalt oxide (LiCoO2, LCO),
-
Lithium manganese oxide (LiMn2O4, LMO),
-
Lithium nickel cobalt manganese oxide (LiNiXMnYCoZO2, NMC),
-
Lithium nickel aluminum oxide (LiNi0.8Co0.15Al0.05O2, NCA).
-
Lithium iron phosphate (LiFePO4, LFP),
-
Lithium manganese phosphate (LiMnPO4, LMP),
-
Lithium cobalt phosphate (LiCoPO4, LCP).
Aluminum foils are used as the current arrester for cathodes. In commercial use, most anodes use graphite as the anode active material [30][31][32][50,51,52] and a thin copper foil as the current arrester.
LIB are designed and built as individual cells. These cells are manufactured in various formats, and a distinction is made between button, cylindrical, prismatic and pouch cells [27][33][47,54]. Cylindrical cells are manufactured in different layers of anode, separator and cathode, which are rolled around a pin to a so-called jelly roll [34][55]. The jelly roll is further integrated into a steel shell, which is filled with the electrolyte, closed and welded [34][55]. Pouch cells are also manufactured with different layers of anode, separator and cathode sheets. These are stacked on top of each other, inserted into the pouch foil, a very thin aluminum-polymer compound, before being filled with the electrolyte and closed [34][55]. For cylindrical cells, the 18650 and 21700 cell formats, in particular, have become established [27][47]. The 4680 cell format is being discussed in particular by American car manufacturer Tesla and will be used there in the future [35][36][56,57]. The 18650 cell format is used for portable batteries in the consumer sector. The dimensions of this format are standardized by the American National Standards Institute in the ANSI C18.1M standard [37][58], while the dimensions of the prismatic and pouch cell formats for automotive applications are standardized by DIN SPEC 91252 [33][54]. Pouch cells used in the consumer sector for products such as laptops, tablets and phones are currently not subject to any standardization. Depending on the application, several cells can be connected in series and/or in parallel in a module [18][20]. Modules use a battery management system (BMS) to manage individual cells. The BMS determines the cell voltage and temperatures, monitors the current, and allows the battery system to be switched on and off [18][20]. For EV batteries, several modules are connected to a battery pack. Current trends also pursue the “cell-to-pack” approach, in which many cells are directly interconnected into a battery pack [38][59].
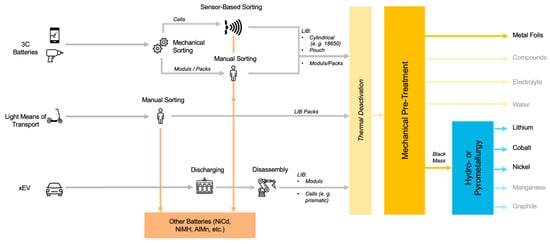
3. Collection and Sorting of Spent Batteries
Since the Battery Directive came into force in 1998, manufacturers and importers of batteries have been required to take back and recycle spent batteries and accumulators free of charge. The legislator provides so-called collection schemes for the return and proper recycling [39][62]. There are various collection schemes in Germany and other European countries, such as the following:- ]

Figure 1.
State of the Art Battery Sorting Concepts (own illustration).
