Radiotheranostics refers to the pairing of radioactive imaging biomarkers with radioactive therapeutic compounds that deliver ionizing radiation. Given the introduction of very promising radiopharmaceuticals, the radiotheranostics approach is creating a novel paradigm in personalized, targeted radionuclide therapies (TRTs), also known as radiopharmaceuticals (RPTs). Radiotherapeutic pairs targeting somatostatin receptors (SSTR) and prostate-specific membrane antigens (PSMA) are increasingly being used to diagnose and treat patients with metastatic neuroendocrine tumors (NETs) and prostate cancer. In parallel, radiomics and artificial intelligence (AI), as important areas in quantitative image analysis, are paving the way for significantly enhanced workflows in diagnostic and theranostic fields, from data and image processing to clinical decision support, improving patient selection, personalized treatment strategies, response prediction, and prognostication. Furthermore, AI has the potential for tremendous effectiveness in patient dosimetry which copes with complex and time-consuming tasks in the RPT workflow.
1. Introduction
Radiotheranostics represents a medical paradigm that uses radiopharmaceuticals for targeted radionuclide therapy (TRT), also known as radiopharmaceutical therapy (RPT). The approach involves the use of the same or different radiopharmaceuticals for both therapeutic and imaging purposes, enabling the matched targeting of specific disease sites
[1][2][3][1,2,3]. The radiotheranostics paradigm enables the visualization of drug pharmacokinetics in the body, enabling personalized medicine frameworks
[1]. Radiotheranostics makes it feasible to customize treatment planning based on individual variations by choosing “the right drug for the right patient at the right time”
[4].
This study specifically concentrates on radiotheranostic ligand pairs that selectively bind to somatostatin receptors (SSTRs) and the prostate-specific membrane antigen (PSMA). In general, PSMA expression is higher in prostate cancer (PCa) cells than benign prostate cells, providing a comparatively specific target for patients with this tumor. Moreover, SSTRs are expressed much higher in neuroendocrine tumor (NET) cells or meningiomas than in normal tissues
[5].
Prostate cancer is one of the three most common cancers in the world (7.1% of all cancers), with a high survival rate (a 5-year survival rate of >95%) and high recurrence rates
[6][7][8][9][6,7,8,9].
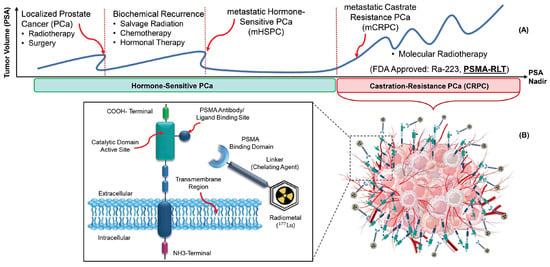
Figure 1A illustrates a simplified disease course for PCa patients. In the first stage, patients are diagnosed through an abnormal serum prostate-specific antigen (PSA) level, a PCa tumor marker. Most of them will be treated (for example, with radiation therapy or surgery), and their PSA will nearly reach zero. However, some of them will have biochemical recurrences. A significant number of PCa patients will progress to metastatic castrate-resistant prostate cancer (mCRPC). Therefore, there is a growing need for alternative therapeutic strategies for these patients. In this regard, several molecules were tested to target PSMA expressed on the cell surface of mCRPC patients
[10].
Figure 1. The principle of radiotheranostics in mCRPC patients. (A). The typical timeline of different therapies, including RPT (also known as RLT). (B). PSMA-binding domain, linker, and chelator labeled with Lu-177 deliver ionizing radiation to the tumor.
PSMA is a type II, 750-amino acid transmembrane protein anchored in the cell membrane of prostate epithelial cells
[11]. Radiopharmaceuticals targeting PSMA for diagnostic imaging purposes include [
68Ga]Ga-PSMA-11, [
68Ga]Ga-PSMA-617, [
68Ga]Ga-PSMA-I&T, [
18F]DCFPyL, [
18F]PSMA-1007 or [
124I]MIP-1095, [
64Cu]Cu-PSMA-617, and [
44Sc]Sc-PSMA-617. For therapies, [
90Y]Y-J591, [
177Lu]Lu-PSMA-617, [
177Lu]Lu-PSMA-I&T, [
90Y]Y-PSMA-617, [
225Ac]Ac-PSMA-I&T, and [
225Ac]Ac-PSMA-617 have been used
[12][13][12,13]. Currently,
68Ga or
18F labeled radioligand binding to PSMA are paramount players in PCa applications
[14].
In March 2022, [
177Lu]Lu-PSMA-617 received Food and Drug Administration (FDA) approval as a treatment option for adult patients with PSMA-positive mCRPC, marketed as Pluvicto
® [15]. The use of radiolabeled PSMA-targeting ligands provides an important theranostic paradigm, with potential for treating mCRPC patients (
Figure 1B)
[16][17][16,17].
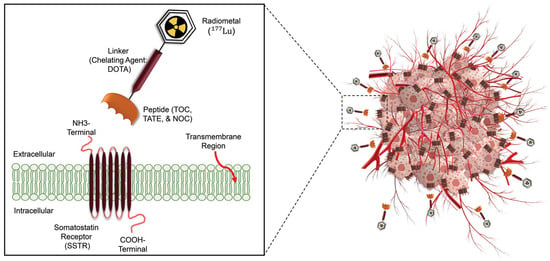
A similar scenario exists for neuroendocrine tumors (
Figure 2). Although the epidemiologic importance of NETs is not as high as that of prostate cancer, NETs consist of 0.5% of all malignancies, and the incidence rate has increased six–fold over the past decades
[18][19][18,19]. Patients with NETs showing high SSTR expression are appropriate candidates for [
68Ga]Ga-/[
177Lu]Lu-SSTR applications
[20].
Figure 2. Schematic overview of radiotheranostics principle in NETs patients and the development of radiopharmaceutical. The chelator labeled with Lu-177 binds to SSTRs and delivers ionizing radiations to destroy tumor cells.
Several PET-labeled peptides, including [
68Ga]Ga-DOTA-Tyr3 octreotide ([
68Ga]Ga-DOTA-TATE), [
68Ga]Ga-DOTA-Phe1 Tyr3 octreotide ([
68Ga]Ga-DOTA-TOC), [
68Ga]Ga-DOTA-1-NaI3 octreotide ([
68Ga]Ga-DOTA-NOC), and [
64Cu]Cu bound DOTA-TATE and DOTA-TOC are synthesized for diagnostic applications. Additionally compounds such as Lutetium-177 (
177Lu), Yttrium-90 (
90Y), and Actinium-225 (
225Ac) bound DOTA-TATE and DOTA-TOC, are produced for therapeutic purposes. In January 2018, the FDA approved [
177Lu]Lu-DOTA-TATE for the treatment of SSTR-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs)
[21]. A complete list of clinically relevant radiotheranostic pairs targeting SSTR and PSMA is shown in
Table 1 [22].
Table 1.
Radiotheranostic pairs and targets in NETs and mCRPC diseases with an emphasis on clinical relevance.
Therapeutic
Radioisotopes |
Diagnostic Radioisotopes-Pharmaceuticals |
| SSTRs Target/NET |
PSMA Target/mCRPC |
| 177Lu |
[68Ga]Ga-DOTA-TATE PET |
[68Ga]Ga-PSMA-617 PET |
| [68Ga]Ga-DOTA-TOC PET |
[68Ga]Ga-PSMA-I&T PET |
| [68Ga]Ga-PSMA-11 PET |
| [64Cu]Cu-DOTA-TATE PET |
[64Cu]Cu-PSMA-617 PET |
| [64Cu]Cu-DOTA-TOC PET |
| No Clinical Match |
[18F]PSMA-617 PET |
| No Clinical Match |
[44Sc]Sc-PSMA-617 PET |
| 225Ac |
[177Lu]Lu-DOTA-TATE SPECT |
[177Lu]Lu-PSMA-617 SPECT |
| [177Lu]Lu-DOTA-TOC SPECT |
| 90Y |
[177Lu]Lu-DOTA-TATE SPECT |
[177Lu]Lu-PSMA-617 SPECT |
| [177Lu]Lu-DOTA-TOC SPECT |
[177Lu]Lu-J591 SPECT |
| [111In]In-DOTA-TATE SPECT |
[111In]In-J591 SPECT |
| [111In]In-DOTA-TOC SPECT |