Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Dennis Holmes.
Needle-biopsy-induced cancer cell displacement is a common event. The risk is influenced by the biopsy technique and the breast cancer type. Evidence suggests that the risk of needle-biopsy-induced cancer cell displacement may potentially increase the odds of local recurrence but has no impact on regional recurrence and long-term survival.
- needle biopsy
- breast cancer
- cryoablation
- seeding
1. Introduction
Minimally invasive breast biopsy or diagnostic needle biopsy is currently the standard of care for obtaining an initial tissue diagnosis of a breast abnormality. The emergence of this standard was driven in part by the common desire to reduce the psychological and esthetic burden, morbidity, and healthcare expense of routine diagnostic breast surgical excisional biopsies of breast abnormalities, the majority of which are likely to be benign [1,2,3][1][2][3]. Consequently, the National Consortium of Breast Centers, the American Society of Breast Surgeons, and the American College of Surgeons quality metrics have established diagnostic needle biopsy (either core needle biopsy or fine needle aspiration) as the most appropriate initial diagnostic approach for breast abnormalities to allow for appropriate selection and pre-operative treatment planning for the minority of patients for whom lesion surgical resection may be appropriate, including those with malignant, high-risk, or discordant pathology or symptomatic lesions [3,4,5,6][3][4][5][6]. Although diagnostic breast excisional biopsy may sometimes be warranted, there is a consensus that diagnostic excisional biopsy should be reserved for the few situations where a needle biopsy might be infeasible due to technical reasons (e.g., the inability to obtain an adequate sample) or patient safety concerns (e.g., underlying coagulopathy) [7].
2. Mitigation or Prevention Measures
Epinephrine-containing anesthetic field block. Common to each of the strategies listed below is the use of a local anesthetic field block containing epinephrine (concentration of 1:100,000–1:200,000 or 5–10 μg/mL). A field block is performed by injecting a local anesthetic solution along all of the margins (near, far, superficial, deep, medial and lateral, and superior and inferior) of the lesion that is to be biopsied. The primary role of epinephrine in the local anesthetic mixture is to cause the vasoconstriction of nearby arteries and veins to restrict blood flow into and out of the area, extend the duration of the pain-relieving anesthetic, and theoretically decrease the potential for the dissemination of cancer cells into the bloodstream [24,25][8][9]. These effects last up to 6 h. Use of a coaxial or introducer. A coaxial or introducer is a large-diameter needle that can be inserted into the breast through which a small-diameter biopsy needle can be inserted to obtain one or more biopsy samples. Coaxial use decreases procedure time, reduces tissue trauma, and reduces tissue contact by isolating the needle tract for insertion and removal of a needle biopsy device multiple times, which theoretically reduces the risk of needle tract seeding. Although there are no breast cancer studies evaluating the impact of coaxial use on the rate of needle tract seeding, data from hepatocellular cancer demonstrate a lower risk of needle tract seeding with the use of a coaxial [26,27][10][11]. Therefore, coaxial use is a reasonable consideration for reducing the risk of breast biopsy needle tract seeding. Fine needle aspiration. Ultrasound-guided or palpation-guided fine needle aspiration (FNA) utilizes a small needle to collect individual or clusters of cells. In general, FNA is performed with a 21–27-gauge needle, a fraction of the diameter of biopsy devices that are typically used for a core needle biopsy (CNB). By collecting a smaller sample of cells, FNA reduces disruption of the tumor mass, decreases bleeding, and potentially lowers the risk of cancer spread. While FNA has its advantages, several limitations are noteworthy. Firstly, while FNA cytology can distinguish benign from malignant lesions, it cannot distinguish invasive cancer from in situ cancer due to its inability to assess tissue architecture in a cytology specimen. Secondly, FNA carries a greater risk of obtaining a non-diagnostic sample due to the relatively small quantity of cells collected with each aspiration. Fortunately, the risk of inadequate sampling can be minimized by having a cytopathologist available at the time of the FNA to rapidly assess sample quality. If the initial sample is non-diagnostic, additional samples can be immediately obtained until an adequate sample has been verified. Core needle biopsy with limited tissue sampling. To ensure adequate tissue sampling, many physicians utilize large-gauge biopsy devices to obtain multiple tissue samples. Often, 5–10 large biopsy specimens are obtained with vacuum-assisted devices, and 3–5 specimens are commonly obtained with automated gun (spring-loaded) devices, though fewer samples are often sufficient. Although there are no direct correlations between core biopsy instrument diameter and the rate of needle tract seeding for vacuum-assisted and spring-loaded devices, technical differences in the biopsy procedure do affect the risk of needle tract seeding. Whereas vacuum-assisted devices are capable of obtaining multiple samples with only a single insertion into the breast, spring-loaded devices require device insertion and removal multiple times to retrieve the specimen and re-load the device after each sampling. Consequently, limiting the number of spring-loaded biopsy needle passes to three or less would reasonably be expected to reduce the risk of cancer cell displacement, especially when combined with a coaxial and an epinephrine-containing anesthetic field block. Cryoablation-assisted needle biopsy. Percutaneous cryoablation is emerging as a minimally invasive alternative to lumpectomy for the management of early-stage breast cancer. Cryoablation utilizes a specialized needle (a cryoprobe) and liquid nitrogen or argon gas to achieve targeted tissue ablation using ultra-low temperatures. Although the typical aim of cryoablation is therapeutic (i.e., complete tumor eradication), cryoablation is playing an increasingly important role in the diagnostic phase of care to facilitate tissue sampling and to minimize the needle tract seeding of cancer cells. Pulmonary medicine provides a perfect example of this, where cryoprobe transbronchial lung biopsy and cryoablation-assisted lung biopsy improve tissue sampling while decreasing complications in the diagnosis of benign and malignant lung conditions [28,29][12][13]. Cryoablation-assisted needle biopsy (CAB) directly addresses the risk of needle tract seeding by enabling ablation of the needle tract immediately after needle biopsy. CAB can be utilized in patients who are willing to undergo lumpectomy, mastectomy, or neoadjuvant chemotherapy, but are unwilling to undergo a diagnostic needle biopsy. CAB can also be utilized to facilitate the needle biopsy of tumors that are poor candidates for therapeutic cryoablation due to large tumor size, locally advanced breast cancer, multifocal/multicentric breast cancer, or other reasons. With the patient positioned supine or supine oblique, a needle biopsy trajectory is chosen that can be used for both the needle biopsy device and the cryoprobe, which ideally is a trajectory that is parallel to the chest wall through the longest horizontal axis of the tumor. Local anesthetic containing epinephrine is then injected as a field block. After creating a dermotomy, the coaxial and needle biopsy device are inserted under ultrasound guidance to the center of the tumor. Ultrasound-guided needle biopsies are obtained, followed by removal of the coaxial. Immediately after withdrawal of the coaxial, the cryoprobe is then inserted under ultrasound guidance through the same needle tract until the tip of the cryoprobe enters the mass. Cryoablation is then initiated with freezing and ablation of the needle tract within and adjacent to the mass, with the goal of ablating the needle tract and a surrounding 2–4 mm radial tissue margin. The duration of each ablation is determined by the cryoablation treatment algorithm, which is usually calculated based on the diameter of the tissue to be ablated. For example, if the diameter of the coaxial/needle tract is 3 mm, then the minimum target diameter of ablation would be 7 mm (3 mm + 2 mm + 2 mm) or greater. A wider cryoablation zone of necrosis would be needed if biopsies were obtained on multiple planes or levels. After completing ablation of the intra-tumor needle tract, the cryoprobe is warmed, and then, withdrawn up to 4 cm (or up to the level of the subcutaneous tissue), at which time a single freeze cycle is repeated to ablate the remaining, unablated segment of the needle tract. Cryoablation-guided needle biopsy. Cryoablation-guided needle biopsy (CGB) is a suitable solution for an individual who has elected to undergo breast cryoablation but is reluctant to undergo a diagnostic needle biopsy due to a fear of needle tract seeding or cancer cell dissemination. CGB makes it possible for the needle biopsy to be performed at the same time as the cryoablation procedure, which eliminates a delay between specimen collection and the tumor ablation. CBG rapidly kills cancer cells that might have been displaced into the needle tract. By enabling rapid freezing of the entire tumor and surrounding margin, CGB obstructs arteries, veins, and lymphatic vessels that are feeding the mass, thereby preventing the shedding of cells into the bloodstream or lymphatic system. The initial steps of CGB and CAB are similar. A needle biopsy trajectory is chosen that is optimal for the cryoprobe as well as the needle biopsy, which is ideally a trajectory parallel to the chest wall through the longest horizontal axis of the tumor. Images are taken and measurements are obtained to select the appropriate cryoablation treatment algorithm. A field block is then created using an epinephrine-containing local anesthetic solution. After making a dermotomy, the cryoprobe is inserted under ultrasound guidance through the center of the visible lesion. With an assistant holding the cryoprobe securely in place, a second dermotomy is created adjacent to the first dermotomy, and the coaxial and needle biopsy devices are inserted under ultrasound guidance directly in line with the cryoprobe, such that the cryoprobe and biopsy needles are positioned on adjacent planes. Ideally, the axis of the biopsy needle would lie immediately anterior or superficial to the cryoprobe. However, the bulky profile of the handpieces of most biopsy instruments and cryoprobes prevents perfect parallel placement of both instruments (Figure 1). Consequently, the pragmatic approach typically calls for the biopsy needle to be inserted at a downward angle immediately adjacent to the cryoprobe, creating a shallow “X”-like orientation of the devices (Figure 1 and Figure 2). With the cryoprobe remaining in position, up to three good quality biopsy specimens are obtained via the coaxial. With both instruments held in place, cryoablation is then initiated before removing the coaxial and biopsy needle containing the final specimen. Iceball growth is then monitored via ultrasound until the biopsy needle is no longer visible within the mass. At this point, the coaxial and needle biopsy device are immediately withdrawn from the breast before both are solidly frozen in place. The cryoablation procedure is continued according to the cryoablation treatment, with the goal of ablating the mass plus a ≥5 mm surrounding normal tissue ablation margin. The cryoprobe is then withdrawn along the cryoprobe tract up to 4 cm or until the final 4 cm of the cryoprobe remains within the breast, assuming that the length of the freeze zone in 4 cm. Cryoablation is then initiated with a single freeze cycle and monitored via ultrasound until the iceball extends >5 mm beyond the axis of the adjacent biopsy needle tract.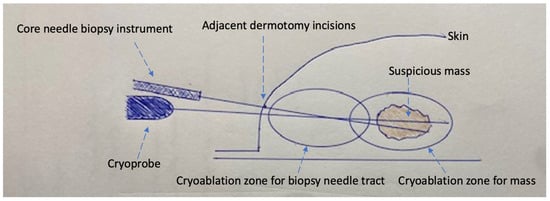
Figure 1. Schematic of cryoablation-guided needle biopsy procedure showing cryoprobe traversing mass, needle biopsy device within mass (coaxial not shown), two adjacent dermotomy incisions, cryoablation zone for the suspicious mass, and additional cryoablation zone for the needle biopsy tract.
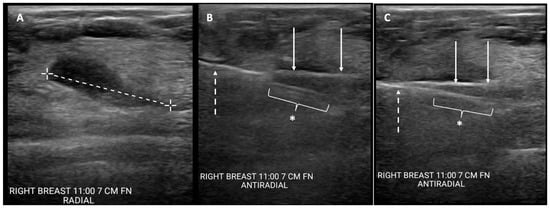
Figure 2. Cryoablation-guided needle biopsy procedure. Ultrasound images showing (A) cancer prior to insertion of devices (broken line indicates longest lesion dimension); (B) cryoprobe inserted through mass (solid arrows) and spring-loaded core needle biopsy device (broken arrow) with aperture open (*), pre-fire; and (C) cryoprobe inserted through mass (solid arrows) with core needle biopsy device (broken arrow) with aperture closed (*), post-fire.
References
- Bruening, W.; Fontanarosa, J.; Tipton, K.; Treadwell, J.R.; Launders, J.; Schoelles, K. Systematic review: Comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann. Intern. Med. 2010, 152, 238–246.
- Gutwein, L.G.; Ang, D.N.; Liu, H.; Marshall, J.K.; Hochwald, S.N.; Copeland, E.M.; Grobmyer, S.R. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am. J. Surg. 2011, 202, 127–132.
- Silverstein, M.J.; Recht, A.; Lagios, M.D.; Bleiweiss, I.J.; Blumencranz, P.W.; Gizienski, T.; Harms, S.E.; Harness, J.; Jackman, R.J.; Klimberg, V.S.; et al. Special report: Consensus conference III. Image-detected cancer: State-of-the-art diagnosis. J. Am. Coll. Surg. 2009, 209, 504–520.
- National Accreditation Program for Breast Centers. Optimal Resources for Breast Cancer. 2024 Standards. NAPBC Guideline. Available online: https://accreditation.facs.org/accreditationdocuments/NAPBC/Standards/Optimal_Resources_for_Breast_Care_2024.pdf (accessed on 26 December 2023).
- American Society of Breast Surgeons. Consensus Guideline on Image-Guided Percutaneous Biopsy of Palpable and Nonpalpable Breast Lesions. Available online: https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Image-Guided-Percutaneous-Biopsy-of-Palpable-and-Nonpalpable-Breast-Lesions.pdf (accessed on 26 December 2023).
- Five Things Physicians and Patients Should Question. Commission on Cancer Choosing Wisely: An Initiative of the ABMI Foundation. Available online: https://www.facs.org/media/wegncq3f/coclist.pdf (accessed on 26 December 2023).
- ACR Practice Parameter for the Performance of Ultrasound-Guided Percutaneous Breast Interventional Procedures. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-GuidedBreast.pdfcomplete (accessed on 26 December 2023).
- Bernards, C.M.; Kopacz, D.J. Effect of epinephrine on lidocaine clearance in vivo. Anesthesiology 1999, 91, 962.
- Schnabl, S.M.; Ghoreschi, F.C.; Scheu, A.; Kofler, L.; Häfner, H.; Breuninger, H. Use of local anesthetics with an epinephrine additive on fingers and penis—Dogma and reality. JDDG J. Dtsch. Dermatol. Ges. 2021, 19, 185–196.
- Maturen, K.E.; Nghiem, H.V.; Marrero, J.A.; Hussain, H.K.; Higgins, E.G.; Fox, G.A.; Francis, I.R. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am. J. Roentgenol. 2006, 187, 1184–1187.
- Fotiadis, N.; De Paepe, K.N.; Bonne, L.; Khan, N.; Riddell, A.; Turner, N.; Starling, N.; Gerlinger, M.; Rao, S.; Chau, I.; et al. Comparison of a coaxial versus non-coaxial liver biopsy technique in an oncological setting: Diagnostic yield, complications and seeding risk. Eur. Radiol. 2020, 30, 6702–6708.
- Dhooria, S.; Sehgal, I.S.; Aggarwal, A.N.; Behera, D.; Agarwal, R. Diagnostic yield and safety of cryoprobe transbronchial lung biopsy in diffuse parenchymal lung disease: Systemic review and meta-analysis. Respir. Care 2014, 61, 700–712.
- Babiak, A.; Hetzel, J.; Krishna, G.; Fritz, P.; Moeller, P.; Balli, T.; Hetzel, M. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration 2009, 78, 203–208.
- Tafra, L.; Smith, S.J.; Woodward, J.E.; Fernandez, K.L.; Sawyer, K.T.; Grenko, R.T. Pilot Trial of Cryoprobe-Assisted Breast-Conserving surgery for small ultrasound-visible cancers. Ann. Surg. Oncol. 2003, 10, 1018–1024.
More
