The complexity of CRISPR-Cas9 applications in GBM research is highlighted, providing unique insights into apoptosis, cell proliferation, and immune responses within the tumor microenvironment. The studies challenge conventional perspectives on specific genes, emphasizing the potential therapeutic implications of manipulating key molecular players in cell cycle dynamics. Exploring CRISPR/Cas9 gene therapy in GBMs yields significant insights into the regulation of cellular processes, spanning cell interphase, renewal, and migration. Researchers, by precisely targeting specific genes, uncover the molecular orchestration governing cell proliferation, growth, and differentiation during critical phases of the cell cycle. The findings underscore the potential of CRISPR/Cas9 technology in unraveling the complex dynamics of the GBM microenvironment, offering promising avenues for targeted therapies to curb GBM growth.
- glioblastoma (GBM)
- gene therapy
- CRISPR
- Cas9
1. Introduction
2. CRISPR/Cas9-Mediated GBM Therapy
Distinctive genetic polymorphisms, ionizing radiation exposure, and the impact of chemical carcinogens on brain cells are among the key pathogenic factors driving the development of GBM [18][46]. Current research is honing in on the promising potential of CRISPR/Cas9 as a cutting-edge gene-editing technology in the realm of immunotherapy for GBM. This innovation is gaining traction in various studies and holds the promise of evolving into a pivotal tool for advancing gene research and engineering strategies in glioma therapy [19][20][47,48].2.1. Targeting Specific Genetic Mutations in GBM
Previous research has not provided a clear classification of precise gene therapy for GBM. Based on the study by Begagić et al. [1], it is observed that the main focuses in GBM therapy are the protein kinase pathway, cell-cycle-related mechanisms, and microenvironmental and immunomodulatory targets. In the realm of CRISPR/Cas9 gene editing for GBM, researchers categorizes specific gene targets into distinct groups, namely: cell cycle regulation, regulation related to the microenvironment, regulation during cell interphase, and targets related to therapy resistance reduction.2.1.1. Cell Cycle Regulation
The cell cycle, a meticulously regulated and intricately orchestrated biological process, stands as a fundamental mechanism governing the growth, development, and maintenance of living organisms [1]. Comprising a series of precisely coordinated events leading to cell division, the cell cycle ensures the accurate transmission of genetic information from one generation of cells to the next. In instances where genetic mutations precede this transmission, the altered information is passed on to progeny cells through the process of cell division. This paradigm is particularly relevant to GBM, as disruptions in the cell cycle regulation, stemming from genetic alterations, contribute to the excessive division and proliferation of neoplastic GBM tissue. Given the strict control exerted by genes over the cell cycle, alterations in these genes lead to dysregulation of cell cycle control mechanisms, fostering uncontrolled division and proliferation of neoplastic GBM cells. Several mutations associated with GBM malignancy have been identified, including those affecting genes such as Epidermal Growth Factor Receptor (EGFR), Erb-B2 Receptor Tyrosine Kinase 2 (ERBB2), Isocitrate Dehydrogenase 1 (IDH1), Neurofibromin 1 (NF1), Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA), Phosphoinositide-3-Kinase Regulatory Subunit 1 (PIK3R1), and Phosphatase and Tensin Homolog (PTEN), among others. The application of CRISPR/Cas9 technology seeks to intervene in the cell cycle of neoplastic cells, aiming to induce apoptosis or autophagy in these aberrant cells, as can be seen in Figure 1.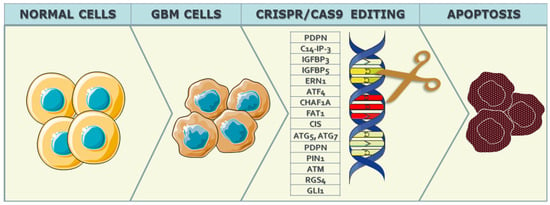
2.1.2. Cell-Interphase-Related Targets
Exploring the realm of CRISPR/Cas9 gene therapy in the context of GBMs, researchers have delved into a diverse array of cell-interphase-related targets to unravel the intricacies of gene regulation during critical phases of the cell cycle. The focus on cell interphase, the period between cell divisions encompassing G1, S, and G2 phases, is crucial in understanding the dynamics of GBM progression and identifying potential therapeutic avenues [30][61]. In recent studies, various genes have been targeted using CRISPR-Cas9 gene editing technology to elucidate their roles in cell proliferation and related functions. Fierro et al. [31][62] focused on PD-L1, employing a knockout strategy to investigate its impact on proliferation, invasion, and macrophage polarization. Lumibao et al. [32][63] targeted CHCHD2, aiming for knockout to understand its influence on mitochondrial respiration, glutathione status, and cell growth inhibition, particularly in the context of EGFRvIII. Toledano et al. [33][64] explored Plexin-A2 through knockout, shedding light on its involvement in cytoskeletal organization, cell flattening, and cell cycle arrest, with a focus on β-galactosidase, MAPK, and FARP2. Gallo et al. [13] delved into the knockout of 14-3-3β, unraveling its effects on proliferation, spheroid formation, and interactions with Bad, FBI1, Raf-1, and Cdc25b. Additionally, Meng et al. [34][65] investigated CDK7, employing a knockout strategy to understand its role in cellular growth. Guda et al. [35][59] targeted RGS4 for knockout, exploring its influence on MMP2 and proliferation. Zhang et al. [36][66] utilized a knockdown strategy for Nanos3, examining its effects on CD133, Oct4, and its implications for proliferation, migration, and chemoresistance. Godoy et al. [37][67] employed knockdown of NRF2, investigating its role in self-renewal and cell proliferation, particularly in relation to SOD. Zhang et al. [38][68] focused on Dazl, utilizing knockout to study its involvement in the CD133/Oct4/Nanog/Sox2 regulatory axis and its impact on proliferation. Lastly, Liu et al. [39][69] explored ERβ through knockout, elucidating its effects on proliferation and apoptosis by targeting ERβ1, ERβ2, ERβ3, ERβ4, ERβ5 (exon 8), mTOR, and STAT-3. Cell renewal studies employing CRISPR/Cas9 technology have investigated specific genes and their roles in regulating crucial aspects of this process. In the work by Bulstrode et al. [40][70], the focus was on Foxo3, utilizing a knockdown approach to assess its impact on differentiation. Specifically, the study targeted FOXG1, SOX2, EGFR, and EGFRvIII in order to delineate their involvement in cell renewal pathways. Saenz-Antonanzas et al. [41][71] explored the role of SRR2 through deletion, aiming to understand its influence on self-renewal capacity with a particular emphasis on SOX2. Additionally, Song et al. (2019) investigated SRSF3 using knockout techniques, unraveling its significance in glioma-associated alternative splicing processes involving SR proteins. Studies targeting cell migration through CRISPR/Cas9 technology have provided valuable insights into the molecular underpinnings of this crucial cellular process. Ogawa et al. [42][73] focused on TP53, employing recombination techniques to explore its influence on migration. Smolkin et al. [43][74] investigated NRP2, Plexin-A4, Plexin-D1, and Semaphorin-3C through knockout strategies, shedding light on their roles in regulating migration processes. Prolo et al. [44][75] delved into MAP4K4 using knockout, elucidating its impact on both migration and invasion. Wang et al. [45][76] explored the knockout of BRG1, revealing its involvement in migration, proliferation, and resistance to TMZ. Shao et al. [46][77] targeted PIK3CD along with PAK3 and PLEK2 for knockout, unraveling their roles in migration and invasion. Chen et al. [47][78] investigated THBS1 and TNF through knockout, shedding light on their contributions to proliferation and migration. Ozyerli-Gokna et al. [48][79] focused on ASH2L and SET1/MLL, employing knockout to understand their roles in both proliferation and migration. Nieland et al. [49][80] targeted miR21 and SOX2 through knockout, providing insights into their contributions to migration, invasion, and proliferation. Lastly, Uceda-Castro et al. [50][81] investigated GFAP, GFAPα, and GFAPδ through knockout, revealing their involvement in invasion processes. In summary, the comprehensive exploration of CRISPR/Cas9 gene therapy in the intricate landscape of GBMs has yielded significant insights into the regulation of crucial cellular processes, spanning cell interphase, renewal, and migration. Through precise targeting of specific genes, researchers have unraveled the complex molecular orchestration governing cell proliferation, growth, and differentiation during critical phases of the cell cycle. The investigations into cell renewal shed light on the roles of Foxo3, SRR2, and SRSF3 in influencing self-renewal capacity and alternative splicing processes. Furthermore, studies elucidating the molecular underpinnings of cell migration, targeting genes such as TP53, NRP2, MAP4K4, BRG1, PIK3CD, THBS1, PD-L1, ASH2L, SET1/MLL, miR21, SOX2, and GFAP, have provided valuable insights into the regulation of migration, invasion, and proliferation in the context of GBMs.2.1.3. Microenvironmental CRISPR/Cas9 Targets in GBM Cells
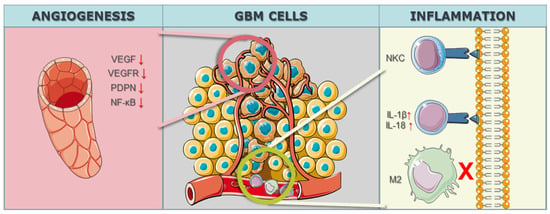
In the realm of GBM research, the intricate modulation of the tumor microenvironment, particularly in the context of angiogenesis, has become a focal point for therapeutic interventions. The study by Han et al. [51][82] targeted the Notch1 gene, employing a knockdown strategy to address hypoxia, angiogenesis, and tumor growth. Notch1 is known for its involvement in diverse cellular processes, and its modulation in the study aimed at disrupting key pathways associated with angiogenesis, a hallmark feature of GBM progression. By utilizing CRISPR/Cas9 technology to downregulate Notch1 expression, the study sought to unravel the intricate interplay between hypoxia, angiogenesis, and the overall growth dynamics of GBM malignant cells. Eisemann et al. [52][83] delved into the role of PDPN, employing a knockout strategy to investigate its influence on the maturation and integrity of the developing vasculature in the murine brain. PDPN, when interacting with C-type lectin-like receptor 2 on platelets, has been implicated in mediating vascular development. By utilizing CRISPR/Cas9 to knockout PDPN, the study aimed to disrupt the finely tuned mechanisms governing vasculature maturation, potentially impeding the vascular support crucial for GBM growth and progression. The targeted gene PDPN serves as a molecular focal point, shedding light on its intricate involvement in orchestrating the vascular microenvironment within the context of GBM. Szymura et al. [53][84] explored the role of DDX39B in regulating the extracellular extracellular matrix (ECM) and promoting angiogenesis through the NF-κB pathway. By employing a knockdown strategy, the study aimed to decipher the contributions of DDX39B in modulating the complex network of signals involved in angiogenesis and ECM regulation. The NF-κB pathway, known for its involvement in various cellular processes, including inflammation and angiogenesis, was specifically targeted to understand its role in the GBM microenvironment. The study adds depth to our understanding of how specific genes can be manipulated to influence the intricate balance of proangiogenic factors in the context of GBM. Continuing the exploration of angiogenesis-related genes, Lu et al. [54][85] investigated the genes BIG1 and BIG2, targeting VEGF through a knockdown approach in 2019. VEGF is a key player in angiogenesis, promoting the formation of new blood vessels to sustain tumor growth. By employing CRISPR/Cas9 to knock down BIG1 and BIG2 and subsequently reduce VEGF levels, the study aimed to disrupt the angiogenic signals that contribute to the vascularization of GBM tumors. The modulation of these specific genes provides insights into the intricate regulatory mechanisms underlying angiogenesis and presents potential avenues for therapeutic interventions aimed at curbing the growth and progression of GBM through microenvironmental control (Figure 2).