You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Kubilay Kaptan and Version 3 by Camila Xu.
Cement, being one of the most widely utilized materials for construction, plays a crucial role as the primary binder in concrete, leading to the formation of a durable, stone-like, hard material capable of withstanding various loads.
- construction and demolition waste
- carbon dioxide reduction
- cement production
1. Introduction
Cement, being one of the most widely utilized materials for construction, plays a crucial role as the primary binder in concrete, leading to the formation of a durable, stone-like, hard material capable of withstanding various loads [1][2][3][4][1,2,3,4].
The conventional kind of cement, known as ordinary Portland cement (OPC), primarily comprises over 90% Portland cement clinker. This particular type of cement is derived from readily accessible raw materials that are widely abundant and cost-effective, making it easily obtainable in nearly all regions [5].
This inexpensive mineral binder has rapid hardening properties in nearly all livable environments, enabling the creation of diverse structures [6]. Moreover, its user-friendly nature allows untrained individuals, including those lacking literacy skills, to utilize it effectively for self-construction purposes [5].
Cement constitutes approximately 10% of the total volume of concrete on a global scale, and approximately 50% of cement is allocated to produce concrete, while the remaining portion is designated for applications such as mortars, pastes, and pre-manufactured products [7].
1.1. Cement Production
1.1.1. Global Cement Production
The global output of cement has witnessed a significant rise over the years. Specifically, it has escalated from 0.94 billion tons in 1970 to 2.284 billion tons in 2005, further increasing to 4.05 billion tons in 2017 and reaching 4.1 billion tons in 2018 [8].
In the year 2017, the countries of China and India, which are recognized as the largest global manufacturers, collectively accounted for 64% of the global cement production. This equated to a total output of 2.61 million tons of cement out of the whole global production of 4.05 million tons [6]. In the year 2019, the primary producers of cement were China, India, the European Union, and the United States [9][10][9,10]. These four entities collectively accounted for 56.1%, 7.8%, 4.4%, and 2.2% of the total cement production, respectively [11].
Current cement consumption is about 4.2 billion tons per year [12], which is enough to produce almost 1.6 m3 of concrete per person. This amount, which is approximately half of the volume of food produced worldwide, is expected to reach approximately 6 billion tons by the end of 2050 [7][13][7,13].
1.1.2. Cement Production Stages
Cement is derived from a combination of limestone, clay, and sand, which serve as the primary sources of lime, silica, alumina, and iron [14]. Cement production by the dry manufacturing process consists of six stages [6][15][16][17][18][6,15,16,17,18].
During the initial phase, the raw materials necessary for the process are extracted through mining operations, such as limestone, clay, laterite, bauxite, iron ore, kaolinite, sandstone, and other similar inorganic materials. All of them, properly dosed, constitute the “Portland clinker crude” (PCC), the last six being in addition the fluxes or mineralizers of the first two (those with the highest dosage), to which, despite their very considerable lower dosage, they reduce their melting point so that they can chemically react more and better and thus form Portland clinker permanently. The second stage of the process entails the characterization of diverse raw materials and their proper dosage to create PCC. During the third stage, the PCC is introduced into a preheating chamber. During the fourth stage, the pre-heated decarbonized PCC is introduced into the rotary kiln to undergo the process of clinkerization at a temperature ≥1450 °C. During the fifth stage, the clinker that emerges from the kiln undergoes a quick cooling process facilitated using pressurized air. During the concluding phase, the cooled clinker is recovered from the cooling vessels and then transferred to the mills. The clinker is ground together with the optimum amount of setting regulator [19] (natural gypsum stone) into powder using a ball mill or roller mill, or a vertical mill, and the pulverized cement is transported to storage silos using a transportation system suitable for shipping (Figure 1). Nevertheless, if the composition of the ground material consists of a combination of natural and/or artificial pozzolans and/or GGBFS along with Portland clinker, the ideal quantity of setting regulator needs to be determined utilizing the R. Talero method [20].
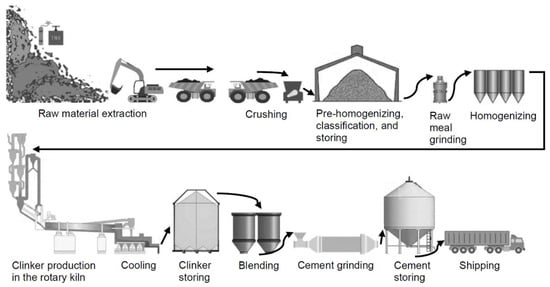
Figure 1.
Cement production.
The six phases can be condensed into three primary stages: raw meal preparation, clinker production, and finish grinding [21][22][21,22].
1.2. Environmental Impacts of Cement Production
In comparison to the year 1750, it has been observed that concentrations of CO2 in the Earth’s atmosphere have risen from 280 to 410 parts per million by volume (ppmV) [23][24][25][26][27][28][29][23,24,25,26,27,28,29]. This upward trajectory is projected to persist in the coming decades, potentially leading to a temperature rise of up to 5.8 °C during the present century [29][30][29,30].
Approximately 40% of worldwide CO2 emissions can be attributed to four key industries: power plants, iron and steel manufacturing, cement manufacturing, and chemicals and petrochemicals [29]. The cement sector is identified as the primary contributor of process emissions [16][31][16,31].
Based on the available worldwide CO2 emission data, cement plants made a substantial contribution of 2.9 billion tons of CO2 in the year 2021 [7]. This figure represents an almost fivefold increase when compared to the emission level of 0.57 billion tons recorded in 1990 [29][32][29,32].
CO2 Emissions from the Cement Industry
Cement production is a highly resource-intensive process that consumes significant amounts of energy and raw materials [16]. This process leads to the emission of CO2 through two primary pathways: direct emissions from the combustion of fossil fuels in the kiln and indirect emissions from the calcination process of the primary raw material, predominantly limestone [33]. Additionally, the consumption of electricity in cement production, particularly when generated from fossil fuel combustion, contributes to overall CO2 emissions [34].
The emission of CO2 during the manufacturing of one metric ton of Portland cement is predicted to range from 0.73 to 0.99 metric tons throughout various geographical regions [34]. It can be asserted that the manufacturing of one kilogram of Portland cement results in the emission of about one kilogram of CO2 into the atmosphere [35][36][35,36].
The global production of this product is responsible for approximately 5–9% of CO2 emissions [13][16][29][37][38][39][40][41][13,16,29,37,38,39,40,41]. Furthermore, it accounts for significant emissions of carbon monoxide (CO) and heavy metals [14]. In addition to CO2, CO, and heavy metals, the use of a substantial quantity of material has led to the excessive burden on deposits of these materials and the alteration of the environment. The production of Portland cement alone entails the consumption of approximately double the quantity of raw materials required to manufacture one metric ton of Portland cement [35].
As previously stated, the process of manufacturing Portland cement results in the emission of carbon dioxide through both direct and indirect means [39]. Indirect emissions are generated because of the calcination process, wherein limestone, the principal constituent of cement, undergoes heating [39][42][43][39,42,43]. The process of thermal decomposition causes the calcium carbonate present in limestone to undergo a chemical transformation, resulting in the formation of calcium oxide and the liberation of CO2 gas [39]. This procedure is responsible for approximately 50% of the total emissions generated during the manufacture of cement [32][41][32,41]. The production of cement involves subjecting limestone and other clay-like materials to high temperatures of approximately 1450 °C within a kiln [39][44][39,44]. Direct emissions arise because of the combustion of fossil fuels utilized for the purpose of heating the kiln, constituting approximately 40% of the total emissions associated with cement manufacturing [5][16][45][5,16,45]. The emissions associated with the quarrying of raw materials, their transportation, grinding processes [46], the electricity consumption for operating additional plant machinery, as well as the packaging and final delivery of cement, all contribute to the remaining 10% of the overall emissions [43][47][43,47].
Furthermore, a range of technological and managerial inefficiencies within the typical cement production process might result in additional CO2 emissions. Geographical location, technological factors, plant and manufacturing efficiency, the energy mix utilized for electricity generation, and the choice of kiln fuels all contribute to additional carbon dioxide CO2 emissions [29][38][39][29,38,39].
1.3. Construction and Demolition Waste
Construction waste results from building constructions and building renovations and consists of surplus material, unusable impaired or fractured material, cut-off pieces, processing waste, worn-out tools and accessories, dismantled shuttering, packaging, and waste produced by construction workers [48][49][50][48,49,50]. On the other hand, after the end of a structure’s life cycle, its demolition is crucial for the growth of cities where inadequate space is the major obstruction. CDW can also be generated in the aftermath of a natural disaster, which presents several significant challenges, such as transportation, storage in an appropriate location prior to processing, and disposal at landfill sites [51].
Overall, it can be stated that CDW is a type of solid waste generated on construction sites and during the entire or partial demolition of buildings and infrastructures [52][53][54][55][56][57][58][52,53,54,55,56,57,58].
1.3.1. CDW Composition and Generation
CDW consists primarily of inert and non-inert materials, such as gravel, concrete, sand, ceramic, tile, metal, plastic, glass, roofing materials, paper, cardboard, etc. The inert waste materials consist of soft and hard inert materials, whereas the noninert waste consists of residual waste and other materials such as metals, wood, plastic, and glass [53][59][60][53,59,60]. Inert fraction waste accounts for between 40 and 85 percent of total waste volume, excluding excavation soils [50][58][61][50,58,61].
It is estimated that the construction industry annually generates more than 3 billion tons of CDW worldwide [62][63][64][62,63,64]. This indicates that CDW accounts for approximately 36% of the world’s total waste production [65]. CDW in the United States rose from 50 million tons in 1980 to 600 million tons in 2018 [61]. More than 1.5 billion tons of CDW are produced annually in China [66][67][66,67], while in the European Union (EU), countries produce about 850 million tons/year, or 31% of the total waste generation in the EU [68].
1.3.2. Environmental Impacts of CDW
The generation of waste results in adverse externalities on the environment, even while a significant portion of CDW consists of inert materials that may not provide as significant a risk as hazardous waste [69][70][69,70]. The disposal of CDW in landfills causes landslides [71], depletes limited landfill resources, exacerbates energy consumption, amplifies greenhouse gas (GHG) emissions, poses public health concerns, and contaminates the environment [44][72][73][74][75][44,72,73,74,75].
In recent years, governmental bodies have enacted new regulations pertaining to the management of waste, encompassing responsibilities, disposal practices, and recycling efforts on a broader scale [76]. Consequently, the urban landscape is undergoing transformation through the establishment of recycling facilities, yet the current recovery rate for CDW remains very low [49][77][49,77]. The expansion of the worldwide population and the concurrent rise in sea levels have resulted in a reduction in the accessible land for dump sites, hence leading to an indirect escalation in the expenses associated with landfills [78].
2. Mitigation and Improvement Measures to Reduce CO2 in Portland Cement Production
Clinker is a transitional product in the production of cement, occurring before the mineral additions (MAs) to create the final cement product. As the temperature rises, the pre-calcined materials undergo physical and chemical transformations, causing them to liquefy and combine, resulting in the formation of lumps [39]. Thus, the manufacturing of cement emits greenhouse gases through both chemical and physical processes. The thermal decomposition of limestone releases CO2 by an endothermic chemical reaction, and the combustion of coal, fuel, or AF releases it as well (but exothermically), only that the transmission to the limestone of the heat generated at the same time, to decompose it and decarbonate it, is not carried out chemically but physically by the following ways: conduction, convection, and radiation. Although it is not possible to completely eliminate these emissions, the use of energy-saving technologies can help reduce physical emissions. Therefore, the cement industry had been actively engaged in the pursuit of techniques aimed at reducing CO2 emissions far in advance of the emergence of global warming as a prominent concern. To address this predicament, an increasing body of research has delved into the process of decarbonization within the cement sector, as outlined in Table 1.Table 1.
Relevant studies on CO
2
reduction methods for the cement industry.
| Reference | Region | Reviewed Methods |
|---|---|---|
| [79][81] | Global | Utilization of Afs/ARMs, supplementary cementitious materials (SCMs), and alternative low-carbon binders. |
| [5] | Global | Improving energy efficiency; use of Afs; clinker substitution by MAs/SCMs; utilization of carbon capture and storage (CCS), alternative clinkers, and alkali-activated materials; and improving the efficiency of cement use. |
| [80][82] | Global | Increased use of calcined clay and engineered filler with dispersants, introduction of new Portland clinker-based cement alternatives, use of alkali-activated materials, and improvement of the efficiency of cement use. |
| [16] | Global | Energy savings and the use of CCS and alternative materials (AFs, ARMs, and clinker substitute). |
| [14] | Global | Improving energy efficiency, material substitution, and the use of AFs and CCS. |
| [81][83] | Global | The use of CCS technologies, reduction of clinker/cement ratio, use of AFs, and pyro-processing improvements. |
| [82][84] | Global | Improving energy efficiency, changing fuel type, the use of CCS, substituting clinker, and improving cement use efficiency. |
| [22] | Global | Reduction of the clinker/cement ratio and the use of ARMs/AFs, energy efficiency improvements, the use of WHR and CCS, and the replacement of cement in concrete or mortar with alternative materials. |
| [83][85] | Global | Utilization of energy conservation approaches. |
| [84][86] | Global | Utilization of CCS, SCMs, and nanotechnology. |
| [85][87] | Global | Utilization of WHR, blended cements, efficiency improvements, and CCS. |
| [86][88] | Global | Utilization of CCS. |
| [10] | Global | Use of low-carbon cement technologies. |
| [87][89] | Asia | Improving energy efficiency, the use of AFs, reduction of the clinker-to-cement ratio, and utilization of emerging and innovative technologies (excess heat recovery, CCS, energy management systems, etc.) |
| [21] | China | The use of energy efficiency improvement technology, WHR, CCS, AFs, and clinker substitution. |
| [88][90] | China | The use of energy efficiency improvements, AFs, clinker substitution, and CCS. |
| [89][91] | China | Utilization of advanced efficiency technologies, ARMs, AFs, renewable electricity, CCS, and cement carbonation effects. |
| [90][92] | China | Use of energy efficiency, AFs, ARMs, and CCS. |
| [91][93] | Indonesia | The use of clinker substitutes, AFs, and WHR and upgrading kilns. |
| [92][94] | Indonesia | Improving energy efficiency and the use of clinker substitution, AFs, and CCS. |
| [93][95] | Japan | The use of energy and material efficiency strategies, AFs, reducing clinker-to-cement ratios, lowering transportation emissions, and decarbonizing electricity supply. |
| [94][96] | Japan | Reuse of building material waste. |
| [39] | Malaysia | The use of energy-efficient technologies, WHR, AFs (fuel switching/co-processing), alternative binders, and CCS. |
| [95][97] | Thailand | The use of WHR. |
| [38] | Hong Kong | The use of Afs and ARMs and the application of combined strategies. |
| [96][98] | USA | Improving energy efficiency. |
| [2] | Portugal | Use of alternative clinker technologies. |
| [97][99] | Poland | Improving energy efficiency and the use of waste as raw materials and MAs in cement production. |
| [98][100] | Italy and Germany | The use of AFs and ARMs. |
| [99][101] | Sweden | The use of CCS. |
2.1. Substitution of Alternative Fuels (AFs)
While AF substitution in the cement production process is not a novel concept [16][100][16,102], its prominence has grown considerably, and the utilization of AFs in cement manufacturing has received significant attention in recent years due to its efficacy in replacing the thermal energy derived from fossil fuels and mitigating pollutant emissions. The contemporary cement kiln exhibits a high degree of adaptability, enabling the cement industry to seamlessly transition between different fuel sources with moderate ease [5][16][5,16]. The cement rotary kiln possesses the capability to incinerate a diverse array of materials because of the extended durations spent at elevated temperatures, the inherent capacity of clinker to assimilate and confine impurities such as heavy metals within itself, and the alkaline conditions prevailing within the kiln [101][103]. The cement industry utilizes conventional fossil fuels, including coal, fuel oil, petroleum coke (petcoke), natural gas, and diesel, in its kilns and pre-heater systems to generate the elevated temperatures required for clinker production [46]. The aforementioned fuels account for over 94% of the thermal energy need in the worldwide cement industry [102][104]. The suitability of AFs is contingent upon various properties, including their physical state (solid, liquid, or gaseous), lower heating value, ash composition and content, toxicity (organic compounds, heavy metals), volatile content [103][105], humidity content, physical properties (scrap size, density, and homogeneity), content of circulating elements, grinding properties, storage/feeding capabilities, and calorific value [15][16][43][103][104][105][15,16,43,105,106,107]. The utilization of AFs offers several key benefits, namely enhanced energy recovery and the preservation of finite fossil fuel resources. These advantages result in the reduction of pollutant emissions, particularly CO2, and a projected decrease in the expenses associated with cement production [16][100][101][106][107][16,102,103,108,109]. Nevertheless, the adoption of AFs presents numerous problems as a result of the complexities associated with integrating supplementary fuel-saving methodologies. Furthermore, it is important to note that not all AFs guarantee a reduction in CO2 emissions due to their elevated carbon intensities [45][79][100][108][109][45,81,102,110,111]. AFs can be broadly categorized into three primary groups [110][112]. The first group comprises liquid AFs, encompassing materials such as waste oil, solvents, animal fat, and sewage sludge. The second group consists of solid AFs, which include waste tires (either chipped or whole), animal and bone meal, dried sewage sludge, scrap wood, and waste materials originating from various industries, such as the pulp, paper, cardboard, plastics, packaging, and textile industries. Lastly, the third group encompasses gas AFs, which encompass landfill gases, pyrolytic gases, and biogases. Typical AFs used by the cement industry include animal meat and bone meat [111][112][113][114][115][116][117][118][119][113,114,115,116,117,118,119,120,121], municipal solid waste [108][120][121][122][123][124][125][126][110,122,123,124,125,126,127,128], refuse derived fuel [127][128][129][129,130,131], waste tires [108][130][131][132][110,132,133,134], plastic waste [22][104][133][22,106,135], saw dust or wood [134][135][136,137], straw [136][137][138,139], agriculture and forest wastes [138][139][140][140,141,142], almond shells [141][142][143,144], olive residues [143][145], oil palm [144][146], food residue [145][147], rice husk ash [146][148], natural gas [147][149], biogas [148][150], sewage sludge [149][150][151][151,152,153], oil sludge [152][154], slaughterhouse residues [153][155], spent solvents [108][110], and solid recovered fuels [154][155][156,157]. It is projected that the global utilization of AFs will increase from 3% in 2006 to around 37% by 2050, resulting in a contribution of approximately 15% towards the intended overall reduction in CO2 emissions [5][34][5,34].2.2. Substitution of Alternative Raw Materials (ARMs)
The process of the decarbonation of commonly used raw materials, primarily limestone, results in the release of around 0.53 metric tons of CO2 for each metric ton of clinker produced [151][153]. Utilizing waste and by-products that include valuable minerals, such as calcium, silica, alumina, and iron, is a viable option to substitute for traditional raw materials, including clay, shale, and limestone [15][156][15,158]. The incorporation of alternative materials into the clinker recipe necessitates a prudent methodology, since any modification in the chemical composition of cement will have an impact on the ultimate quality of the product [22][157][22,159]. Various industrial by-products and waste-derived materials have been investigated as potential substitutes for limestone and clay in the production of cement. The objective is to minimize the utilization of natural resources, decrease CO2 emissions, and reduce heat consumption while ensuring that the manufacturing processes remain unaltered [157][159]. Some of the ARMs utilized in the raw meal for cement production are presented in Table 2.Table 2.
Typical ARMs utilized as a partial replacement in the raw meal.
2.2.2. Substitution of CDW as an ARM
The chemical and mineralogical properties of CDW are sufficient to qualify it as a viable substitute raw material in the limestone–clay mixture produced during the manufacturing process of Portland clinker. The composition of CDW typically includes calcium, silicon, aluminum, iron, and several trace elements, including magnesium, potassium, titanium, and sulfur. These minor elements have the potential to contribute to the development of the primary phases of Portland cement [161][167][178][288][289][163,169,180,290,291]. Furthermore, the substitution of CDW leads to a decrease in the generation of CO2. This waste serves as a source of CO2 that is separated from calcium oxide (CaO), thereby reducing the decarbonation of limestone that occurs during the flaring process in the manufacturing of clinker [161][178][163,180]. From the above-mentioned ARMs, CO2 emission related studies concerning CDW are listed in Table 3.Table 3.
CO
2
emissions for CDW substituted in the raw meal.
Table 4.
Minerals used as an addition or partial replacement for Portland cement to produce concrete.
| MA | Mix Type | Optimum Substitution (wt.%) | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [ | 163][164][165][166][167][26,165,166,167,168,169], cement waste [168][169][170][170,171,172], recycled aggregates (RAs) [171][172][173,174], marble and brick waste [173][175], cement kiln dust [174][175][176,177], ceramic wastes [176][177][178][178,179,180], recycled mortar or paste [179][181], cellular concrete [180][182], asbestos cement tile waste [181][183], inorganic construction waste [182][184], dam fine sediments [183][184][185,186], and dredged sediments [185][187[186],188]. | |||||||||||
| Hydrated cement waste (HCW) | HCW is obtained as a by-product from the efficient separation of fine recycled concrete aggregates. CWp-A is prepared by replacing 30% weight of ordinary Portland powder by HCW. CWp-B is prepared with a higher amount of HCW, 55% in weight. | OPp | 76.00 | - | 24.00 | - | Significant reductions in CO | Manufacturing Industry |
Sewage sludge | |||
| Agricultural Industry | 2 | emissions connected with clinker/cement production are reported in both scenarios (low or high amounts of HCW). | ||||||||||
| [ | 187] | |||||||||||
| −5 | , 2.41 × 10 | −5 | , 2.3 × 10 | −5 | , 2.2 × 10−5, and 2.1 × 10−5 respectively. | . | [188] | CWp-A[189][190][191][192][193][194][189,190,191,192,193 | 53.00 | - | 17.00 | 30.00 |
| Sugarcane bagasse ash (SCBA) | ,194,195,196], blast furnace slag [195][196][197][198][199][200][197,198,199,200,201,202], lime sludge [13][201][202][13,203,204], steel slag [199][203][204][ | Ordinary concrete | <25 | |||||||||
| [365][366] | ||||||||||||
| [427 | 201,205,206], stainless steel slag [205][207], basic oxygen furnace slag [206][207][208,209], calcium carbide slag [208][210], magnesium slag [209][211], water purification sludge [210][212], heavy metal-containing sludge [211][213], electric arc furnace slag [212][214], fly ash [195][196][198][213][214][215][216][217][218][219][220][197,198,200,215,216,217,218,219,220,221,222], red mud [197][221][222][223][199,223,224,225], oil-based mud [224][225][226,227], iron ore tailings [226][227][228,229], copper tailings [228][230], industrial hazardous waste [229][231], paper pulp waste [230][231][232,233], marine bio-refinery waste [232][234], glass waste [38][233][38,235], plastic waste [234][236], fiber-cement waste [235][237], black dross leached residue [236][238], and titanium dioxide waste [237][239]. | |||||||||||
| [ | 297 | CNSA | ][298][299][ | Ordinary concrete300][301],303[302],304[303],305[304],306[305],307[306][299,300,301,302,308] | 5, 10, 15, 20 | 10 | The embodied carbon of 5%, 10%, 15%, and 20% CSA is 4%, 7%, 11%, and 15% lower than that of the control mix. | Agricultural and Aquacultural Industries |
Wood ash [238][239][240,241], biomass ash [108][110], sugar filter mud [240][241][242,243], pulverized eggshell waste [242][244], bone ash [119][121], and pulverized oyster and scallop shell waste [243][245]. | |||
| Natural sources | Basalt rock [102][106][244][104,108,246], natural fluorapatite [245][247], meta-schist [246][248], Callovo-Oxfordian argillite [247][249], spent volcanic soil [18], calcined clay [248][249][250,251], and spent limestone sorbent [250][252]. | |||||||||||
| Other sources | Municipal solid waste [251][252][253][253,254,255], contaminated soil [254][256], and mining waste [255][257]. | |||||||||||
2.2.1. Consideration of CC as SCM: Replacement of Portland Cement by CC
The materials evaluated in Table 2 have the potential to partially substitute for Portland clinker by means of novel variations of already utilized SCMs. Among these materials, calcined clay (CC) deserves particular attention. By subjecting ordinary clay, which typically contains at least 40% kaolinite and is widely available in the earth’s crust, to moderate heat treatment (about 700 and 850 °C), it can be transformed into a pozzolanic material called CC [256][257][258,259]. CCs, especially when combined with limestone, are being recognized as a highly promising solution due to their excellent performance and the abundance of sufficient reserves of these materials [258][260]. Limestone calcined clay (LC2) and limestone calcined clay cement (LC3) systems exploit the synergistic effects of calcined clay and limestone, enabling a significant decrease of up to 50% in the utilization of clinker [259][261]. Nevertheless, the clays typically employed in LC3 systems consist of a minimum of 40% kaolinite [256][260][258,262]. Recently, there has been a significant increase in research [261][262][263][264][265][266][267][268][269][263,264,265,266,267,268,269,270,271] focused on the potential utilization of CC as an SCM in the manufacturing of cement, with a particular emphasis on advancing its economic viability [270][272]. Zhu et al. [271][273] conducted a study on the characteristics of LC2 blended cement and compared them with fly ash (FA) and granulated blast-furnace slag (GGBS). They reported that the normal consistency of LC2 blended cement was greatly raised and the substitution of LC2 at a rate of 60% resulted in an almost twofold increase in normal consistency. Dhandapani et al. [272][274] reported that concrete produced with LC3 had superior compressive strengths compared to concrete with equal combination proportions at all ages up to 1 year. The investigation carried out by Vaasudevaa et al. [273][275] involved the substitution of cement in concrete with a combination of LC2 at a proportion of 45%. They concluded that the compressive strength of steam-cured LC3 concrete after 1 day is comparable to that of OPC concrete, exhibiting a similar strength enhancement resulting from the steam curing conditions. The study carried out by Aramburo et al. [274][276] aimed to evaluate the mechanical properties and sulfate resistance of blended cements containing a significant amount of CC as pozzolanic material. The objective was to demonstrate that these cements can meet the requirements of CEM type IV/A-SR and IV/B-SR cements as defined by the EN 197-1:2011 standard. The results obtained validated the increase in sulfate resistance and the decrease in the mechanical strength of PC when it was replaced by CC (whose matrix clay was kaolin doped with ≈50% quartz) in quantities greater than 40%. They also stated that the blended cements with high percentages of CC replacement successfully met the specified requirements regarding compressive and flexural strengths without prejudice to its decrease observed with the increase in its replacement by PC. The reason for both opposing behaviors, sulfatic and mechanical strengths, was the same: the very high, early, and fast pozzolanic activity of its silica and reactive alumina contents especially (38.0% and 15.0%, respectively) [275][276][277][277,278,279], which excessively decreases the [Ca (OH)] in the liquid phase of its pastes. To verify this, the authors repeated the tests, replacing a small portion of the CC used with slaked lime powder (calcium hydroxide, Ca (OH)). Both behaviors contrasted again, but in the opposite direction; that is, the sulfate resistance decreased, and the mechanical strengths increased, as when the replacement by PC was ≤40%. This was similar to how it also increased its resistance to carbonation, which had also been significantly diminished and seriously compromised, with an increase in the replacement of CC by PC. The more impaired the material, the greater the 40% replacement was [278][280]. A study carried out by Yu et al. [279][281] investigated the practicality of creating a cost-effective and environmentally friendly cement by combining LC2 at a significant proportion of 50–80% relative to the weight of the cement. They reported that blended cements containing 50–60% LC2 exhibit satisfactory compressive strength, decreased hydration heat, reduced environmental effect, and lower material cost per unit strength but reduced workability in comparison to plain Portland cement. This contrasts quite a bit with the results of flexural and compressive strengths obtained by Arámburo et al. [274][276]. With regard to CO2 emissions, a review of the existing literature [80][258][265][279][280][281][282][283][284][285][286][| ] | ||||||||||||||||
| [ | ||||||||||||||||
| 428] | Biomass fly ash (BFA) and coal fly ash (CFA) | Ordinary concrete | BFA/CFA: 10/10, 20/20, 30/30 | 30/30 | GWP impact values (kg CO2eq) are 7.84 × 102 | |||||||||||
| CWp-B | 25.00 | - | 20.00 | 55.00 | ||||||||||||
| for the control mix, 6.62 × 10 | 2 | for 10/10, 5.38 × 10 | 2 | for 20/20, and 4.15 × 10 | Eco-friendly concrete | |||||||||||
| 2 | for 30/30. | [421][<30 | 422] | |||||||||||||
| [428][ | [ | 307][308][309][309,310 | RHA,311] | Calcium aluminate cement concrete |
2.5, 5, 7.5, 10 | 5 | 429]5%RHA could reduce CO2 emissions by 18.75%. | SCBA and CSA | Ultra-high-strength concrete | SBA/CSA: 10/2, 20/2, 30/2, 10/4, 20/4, 30/4, 10/6, 20/6, 30/6, 10/8, 20/8, 30/8 | 20/4 | Considering the cost/MPa, the results show that the use of 20/4 had a higher lower cost per m3 in comparison with all concrete mixture. The reduction in concrete cost was 18.50% compared to the control mix. | [44] | Civil construction waste (CCW) | Reusable or recyclable aggregate waste materials, such as soil from earthworks, bricks, tiles, cladding plates, mortar, concrete, and curbs, are used for CCW. CCW0–10: concrete (1%), mortar (47%), rock (2%), ceramic (13%), and soil (37%); CCW10–20: concrete (41%), mortar (39%), rock (13%), and ceramic (7%); CCW20–40: concrete (57%), mortar (34%), rock (7%), and ceramic (2%). | C-REF |
| [ | 93.20 | 6.80 | - | 328.00 | 500.00 | - | - | |||||||||
| Self-compacting concrete | 15 | |||||||||||||||
| [305][307]429][430] | [ | 310][312] | SDA | Ordinary concrete | 5, 10, 15, 20 | <20 | Embodied carbon (kg CO | Corn cob ash (CCA) and glass powder (GP) as binary cementitious material (BCM)2 | Ordinary concrete | CCA/GP: 2.5/2.5, 5/5, 7.5/7.5, 10/10 | 5/5/kg) for SDA is 0.0014. The embodied carbon of concrete mixtures incorporating 20% SDA is approximately 20% lower than that of the concrete mixtures incorporating PC as the only binder. | Concrete mixtures incorporating 5%, 10%, 15%, and 20% BCM as partial replacement of Portland cement have 4.3%, 8.3%, 12.7%, and 16.8% lower embodied carbon control than the mixtures without BCM. Similarly, the incorporation of BCM into the mixtures led to a reduction of approximately 21% in the embodied energy of the concrete. | C-CCW-1 | 85.71 | - | |
| [305] | 14.29 | 326.00 | 488.00 | 0.60 | 2.40 | |||||||||||
| Ultra-high-strength concrete | [15–30 | 307][311][312][313][313,314,315] | SCBA | Portland fly ash cement concrete |
50, 60, 70 | 50 | ||||||||||
| [391][392] | Mixed cathode ray tubes (CRT) and mixed-container glass (MRF) | The CO | 2 | Ordinary concrete | MRF/CRT: 17/3 | 17/3-eq intensity values of control mix, BA50, BA60 and BA70 concretes were 9.65, 6.17, 6.73, and 7.67 kg CO | The GWP value is 1040 kg CO2-eq. for the control mix and 849 kg CO2-eq for 17/3. | C-CCW-4 | 89.53 | - | ||||||
| Rice husk ash (RHA) | Ordinary concrete | 10–25 | [314 | |||||||||||||
| [341] | ] | [ | 315][ | 10.47 | 316318.00 | 471.80 | []3.00 | 5.60 | ||||||||
| 342] | [ | 317 | ][318][80,316, | POFA and ESP317, | Eco-friendly structural foamed concrete318,319] | POFA/ESP: 20/5, 20/10, 20/15, 25/5, 25/10, 25/15 | 25/5 | CO2 emissions (kg CO2/m3) are 453.97 for control mix, 358.29 for 20/5, 339.61 for 20/10, 320.93 for 20/15, 339.04 for 25/5, 320.36 for 25/10, and 301.68 for 25/15. | C-CCW0–10 | 90.14 | - | 9.86 | 312.00 | 459.50 | ||
| Eco-friendly concrete | 4.90 | |||||||||||||||
| [430 | 5–15 | ][431][319][320 | 8.10 | |||||||||||||
| ] | [ | 321][322][323][320,321,322,323,324] | Cane bagasse ash (CBA) and waste glass (WG) | Green concrete | CBA/WG: 15/5, 10/10, 5/15 | 15/5 | Replacement of 20% of cement with CBA and WG showed reductions in CO2 emissions of about 20% compared to control mix. | C-CCW10–20 | 90.90 | - | 9.10 | 324.00 | ||||
| Self-compacting concrete | 5–15 | [324][325][326][325,326,327 | 488.00 | ] | 1.20 | 2.40 | ||||||||||
| C-CCW20–40 | 90.50 | - | 9.50 | 325.00 | 488.00 | |||||||||||
| Ultra-high-performance concrete | 20 | 0.90 | [327 | 2.40 | ||||||||||||
| ] | [ | [181][183] | Asbestos cement tile waste (ACW) | ACW in the form of aged tiles extracted from a roof. | CL-AC0 | 94.53 | 5.47 | - | 335.00 | 503.76 | - | - | ||||
| CL-AC24 | 72.05 | 3.94 | 24.01 | - | - | - | - | |||||||||
| CL-AC49 | 48.82 | 2.36 | 48.82 | 319.01 | 468.45 | 4.77 | 7.00 | |||||||||
| CL-AC74 | 24.82 | 0.72 | 74.46 | 303.06 | 434.84 | 9.53 | 13.68 | |||||||||
| CL-AC86 | 14.24 | - | 85.76 | - | - | - | - |
2.3. Replacement of MAs in Portland Cement
Due to the production of GHGs, a majority of concrete mixtures use SCMs either through the use of blended cements or by individually adding them to the mixer [215][217]. The incorporation of low-embodied carbon and low-energy elements in the substitution of Portland cement can significantly diminish the overall environmental consequences of binders and, as a result, of concrete [157][291][159,293]. These materials are commonly known as MAs or SCMs. When they are included into concrete and mixed with Portland cement, they create cementitious particles. However, on their own, they do not contain any cementitious compounds [215][217]. The selection of MAs for substituting Portland cement is contingent upon the geographical area and the specific solid waste or byproducts produced by industries or the presence of naturally occurring minerals in these regions [37]. The utilization of MAs as substitutes for Portland cement in concrete offers various sustainability benefits. MAs typically consist of industrial waste products, natural pozzolans, and activated minerals that possess either hydraulic or pozzolanic characteristics. When MAs are used alone or in contact with water, they generally do not exhibit substantial hydraulic reactions that contribute to the cementitious properties. Nevertheless, when exposed to alkaline aqueous conditions or in the presence of calcium hydroxide, fine particles undergo a chemical process known as the pozzolanic reaction. This reaction leads to the formation of hydration products that resemble those seen in Portland cement systems [198][292][293][200,294,295]. A wide variety of materials are available for use as MAs, including natural MAs (volcanic materials, including tuffs, ashes, pumicites, perlites, zeolites, etc.), calcined natural MAs (calcined kaolinite clay or metakaolin), LC3 materials (limestone calcined clay cement), by-product materials (agricultural wastes, CDW, ashes, glass, ferrous slags, non-ferrous slags, basic oxygen furnaces, and electric arc furnaces) [198][293][294][295][296][200,295,296,297| 2 | ||||||||
| M-3/MPa, respectively. | ||||||||
| [ | ||||||||
| 316 | ||||||||
| ][317] | Ultra-high-performance concrete | |||||||
| [309][311] | SCBA and SF | Ecofriendly ternary concrete |
SCBA/SF: 10/10, 20/20, 30/30, 40/40, 50/50 | 30/30 and 20/20 | ||||
| 328 | ] | [328,329] | ||||||
| Pervious concrete | 10–15 | [329][330] | ||||||
| Recycled aggregate concrete | 20 | [330][331][331,332] | ||||||
| Wood waste ash (WWA) | Ordinary concrete | 10 | [332][333][334][335][336][333,334,335,336,337] | |||||
| Self-compacting concrete | 10 | [337][338] | ||||||
| Palm oil fuel ash (POFA) | Ordinary concrete | 10–20 | [338][339][340][339,340,341] | |||||
| Eco-friendly structural foamed concrete | 25 | [341][342] | ||||||
| Lightweight concrete | 10–15 | ][342][343 | Nano silica (NS)] | |||||
| High-strength concrete | 1, 2, 3 | 2 | The climate change index for reference concrete is 534.26 kg CO | 2 | eq. The climate change index for HSC-NS1, HSC-NS2, and HSC-NS3 is 438.55, 426.70, and 415.56, respectively. | Sustainable lightweight foamed concrete | 20 | [343] |
| [347][348] | [ | 344] | ||||||
| POFA | Self-compacting concrete | 50, 60, 70 | 50–70 | The concrete specimens have up to 32–45% reduced carbon dioxide emissions. | Sustainable foamed concrete | 15 | ||
| [342][343] | [ | 344][345] | ||||||
| Lightweight concrete | 5, 10, 15, 20, 25 | 10–15 | Total CO | 2 emission values for mixes M0, M5, M10, M15, M20, and M25 were 0.477, 0.454, 0.430, 0.407, 0.384, and 0.361 CO2-e/m3, respectively. | ||||
| [425][426] | Limestone | Ordinary concrete | 35–65 | <50 | The production of concretes made of limestone-rich cements exhibited roughly 25% less CO2 emissions. | |||
| [396][397] | Self-compacting concrete | 15, 25 | <25 | For control mix, CO2-eq is 5.69 × 102 kg/m3. For 15% and 25% replacement levels, CO2-eq is 4.87 × 102 and 25 4.34 × 102, respectively. |
Table 6.
CO
2
reduction through partial replacement of Portland cement with AM (ternary blended cements).
| Reference | MAs | Mix Type | Amounts of Substitution (wt.%) | Optimum Substitution (wt.%) | Results for CO2 Emissions | ||||
|---|---|---|---|---|---|---|---|---|---|
| [426][427] | Brick dust (BD) and LP | Plain cement concrete | BD/LP: 15/5, 10/10, 7/13, 5/15 | 15/5 | Using PL and BD can save costs of cement in the range of 7–12.5%, which eventually reduces CO2 | ||||
| The use of ETC concretes has a very significant sustainability impact by contributing to the reduction in CO | |||||||||
| 2 | |||||||||
| emissions caused by Portland cement. | |||||||||
| [ | |||||||||
| 431][432] | Limestone filler (LSF), calcined orange illitic clay (OIC), natural pozzolan (NP) and GGBS | Ordinary concrete | LSF/OIC: 20/7.5. LF/NP: 12.4/12.6. LF/GGBS: 6/22; 11/11 | 20% of LF | CO2 emissions (kg CO2/m3 | ||||
| Self-compacting concrete | |||||||||
| <70 | |||||||||
| [ | |||||||||
| 345 | |||||||||
| ] | |||||||||
| [ | |||||||||
| 346 | ] | [347][348][346,347,348,349] | |||||||
| ) for control mix is 399.8, 378.6 for 20/7.5, 380.6 for 12.4/12.6, 322.6 for 6/22, 341.7 for 11/11. | Self-consolidating high-strength concrete | <50 | [349][350][350,351] | ||||||
| Structural lightweight aggregate concrete | 37.5 | [351][352] | |||||||
| Recycled aggregate concrete | |||||||||
| [396][397] | FA and LP | Self-consolidating concrete | FA/LP: 30/15, 40/15, 50/15, 60/15, 20/25, 30/25, 40/25, 50/25 | <50% | CO2 | 20 | [330][331][331,332] | ||
| -eq (kg/m | 3 | ) for control mix is 5.69 × 10 | 2 | , 3.33 × 10 | 2 for 30/15, 2.82 × 102 for 40/15, 2.32 × 102 for 50/15, 1.83 × 102 for 60/15, 3.32 × 102 for 20/25, 2.81 × 102 for 30/25, 2.31 × 102 for 40/25, 1.82 × 102 for 50/25. | Palm oil clinker powder (POCP) | Environmentally friendly lightweight concrete | 15 | [352][353] |
| Lightweight concrete | 15 | [353][354] | |||||||
| Recycled aggregate concrete | 15 | [330][331][331,332] | |||||||
| Eggshell powder (ESP) | Ordinary concrete | 10–15 | [354][355][356] | ||||||
| [ | |||||||||
| 332 | |||||||||
| ] | [ | 333] | |||||||
| Sawdust ash (SDA) | Ordinary concrete | 5–20 | [360][361][361,362] | ||||||
| 20, 40, 60, 80 | Self-compacting concrete | 10 | [362][363[363],364] | ||||||
| Coconut shell ash (CNSA) | Ordinary concrete | 10 | [364][365[365],366] | ||||||
| 60 | Wheat straw ash (WSA) | Ordinary concrete | 5 | [316][317] | |||||
| Nano-POFA | Ordinary concrete | 10–20 | [366][367] | ||||||
| Lightweight concrete | 15 | [353][354] | |||||||
| Nano-POCP | Semi-lightweight concrete | 10 | [367][368] | ||||||
| The best environmental assessment results occur when the SCBA substitution rate is 80%. The global warming potential data decreased by 17.47%. | Nano-ESP | Ordinary concrete | 12.5 | [366][367] | |||||
| High-strength concrete | 5 | [368][369] | |||||||
| Aquacultural Industry | |||||||||
| Seashell powder (SSP) | Ordinary concrete | 5–15 | [369][370][371][372][370,371,372,373] | ||||||
| High-strength concrete | 5 | [373][374] | |||||||
| Oyster shell powder (OSP) | |||||||||
| [381][382] | Ceramic | Ultra-high-performance concrete | 15, 25, 35, 45, 55 | Ordinary concrete | 5–15 | [374][375] | |||
2.4. Substitution of CDW as a MA
Concrete, masonry, and brick wastes are prominent among the various waste fractions, exhibiting a significant proportion of approximately 80% in the overall global production of CDW [66][436][437][438][66,437,438,439]. Researchers have proposed the recycling of this prominent part to serve as a viable solution to address the sustainability issues encountered by the concrete industry [71][439][440][441][442][443][444][445][71,440,441,442,443,444,445,446]. The recycling process involves the conversion of CDW into a reduced-sized fraction through the utilization of mobile or fixed recycling plants [446][447]. The recycling process of CDW primarily results in the production of three distinct fractions [82][447][448][449][84,448,449,450]. One of these fractions includes a range of 25.00–5.00 mm, which is classified as recycled coarse aggregate (RCA). Another fraction falls within the range of 5.00–0.15 mm and is referred to as recycled fine aggregates (RFA). Lastly, there is a fraction that measures less than 0.15 mm, known as recycled powder (RP). It is important to highlight that in addition to the production of recycled coarse and fine aggregates, a significant quantity of fine recycled powder (RP), comprising approximately 15–35% of the total processed CDW mass, is generated [447][448][448,449]. This fine powder lacks a suitable destination and is typically disposed of in landfills [440][450][441,451]. The particulate matter emanating from cement mortar, concrete, or bricks typically has a fine texture. The observed range of diameters for the hybrid powder obtained from the crushing and sieving location of CDW was found to vary between 45 and 150 μm [440][441]. Although the application of RCA has gained increasing popularity in the past years, the possible use of RP as a partial replacement for Portland cement in concrete has received significant attention due to its tiny particle size and consequential reactivity [451][452]. Nevertheless, the efficacy of RPs is contingent upon their primary sources, which are impeded in their practical implementation due to their intricate components. When comparing RPs to Portland cement, it is observed that RPs exhibit a greater degree of irregularity and roughness in their shapes. Additionally, the little particles tend to cluster on the larger ones, resulting in a higher water consumption requirement to obtain a desired standard consistency [438][451][439,452]. The primary factor impeding the utilization of untreated RP derived from CDW in cementitious materials is its inherent low activity. The untreated powder is primarily comprised of inert hydrated materials, namely quartz or calcite [438][444][439,445]. Several modification approaches have been devised to enhance the characteristics of untreated RP, including mechanical activation [452][453][453,454], CO2 curing treatment [454][455][456][457][455,456,457,458], thermal treatment [444][458][459][460][445,459,460,461], tannic acid treatment [461][462], and chemical activators [462][463]. CDW-based material additions used as an addition or as a partial replacement of Portland cement to produce concrete are presented in Table 7.Table 7.
Substitution of CDW in Portland cement to produce concrete.
| Reference | CDW Type | Mix Type | Materials Used in the Mix | Treatment Method | Particle Size or Median Particle Size of CDW (d50) | Amount of Substitution (wt.%) |
|---|
Table 8.
Chemical properties of cementitious materials used (%).
| Reference | Cementitious Material Type | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | Loss-on-Ignition (LOI) |
|---|---|---|---|---|---|---|---|---|---|---|
Table 9.
CO
2
reduction by the partial replacement of Portland cement with CDW.
| Reference | Label | Proportions | w/b a | SP b | 28 d Compressive Strength (MPa) | CO2 Emission (kg/m3 | Optimum Substitution (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ) | Global Warming Potential (GWP) | ||||||||||||||||
| [463][464] | Dehydrated cement paste (DCP) | Green ultra-high-performance concrete | Cement (PO 52.5), DCP, LP, SF, sand, superplasticizer (SP). | Heating | |||||||||||||
| [463][464] | |||||||||||||||||
| [ | OPC | <75 μm | 12.5, 25, 37.5, 50 | <25 | |||||||||||||
| 463][ | 19.383 | 464 | 4.581 | 3.282 | ] | 63.074 | 2.786 | 0.175 | 1.027 | c | 3.498 | 1.540 | |||||
| DCP0 | Control mix | 0.5 | 33.00 | 105.00 | 377.00 | 1.000 | [443][444] | RP | Ordinary concrete | Cement (OPC), RP, natural coarse aggregate (NCA), natural fine aggregate (NFA). | Repeated recycling | <150 μm | 10, 20, 30 | 10–20 | |||
| DCP | 19.967 | 4.997 | 4.125 | 62.405 | 1.849 | 0.137 | 0.781 | 2.949 | 2.261 | [464][465] | Ground recycled concrete (GRC) | Structural concrete | Cement (OPC), GRC, mixed recycled CDW aggregate. | NA | Not stated | 10, 25 | 10 |
| [464][465] | OPC | 18.700 | 5.100 | 2.600 | 65.100 | 1.800 | 0.200 | 0.500 | 3.000 | 2.500 | [465][466] | Recycled brick powder (RBP) | |||||
| GRC | 46.100 | Ultra-high-performance concrete | Cement (PII 52.5R), RBP, SF sand, SP. | NA | 3.800 | 1.500d50: 9.8 μm | 15, 30, 45 | 15 | |||||||||
| 40.000 | 0.500 | 0.300 | 1.200 | 0.400 | 6.200 | [438][439] | RP | Ordinary concrete | Cement (OPC), RP, FA, sand, NCA, water reducing agent. | NA | |||||||
| [466][467] | WBP | 36.510 | 23.440 | d50: 9.06 μm | 15, 30, 45 | 15–30 | |||||||||||
| 15.140 | 4.530 | - | - | 1.510 | - | 4.520 | [466][467] | Waste brick powder (WBP) | Ordinary concrete | Cement (OPC-Grade C-53), WBP, natural aggregate (NA), sand. | NA | <75 μm | 5, 10 | ||||
| [467][468] | 10 | ||||||||||||||||
| OPC | 23.770 | 4.960 | 4.130 | 60.320 | 2.680 | 0.320 | 0.620 | 2.260 | 2.380 | [467][468] | Recycled concrete powder (RCP) | Green ultra-high-performance concrete | Cement (P.II 52.5R), RCP, SF, sand, SP. | NA | d50: 12.04 μm | 15, 30, 45 | 30 |
| Green concrete | |||||||||||||||||
| RCP | 39.830 | 12.500 | 6.010 | 18.660 | 1.970 | 0.850 | 2.340 | 2.040 | 16.750 | [468][469] | RP | Green concrete | |||||
| [468 | Cement (PO 42.5), RP (brick powder and concrete powder), NA, RA, river sand, SP. | ][469] | OPC | 19.240 | 4.080 | 3.250 | 62.470 | ||||||||||
| <20 | |||||||||||||||||
| [ | |||||||||||||||||
| 375 | |||||||||||||||||
| ] | |||||||||||||||||
| [ | |||||||||||||||||
| 376 | |||||||||||||||||
| ] | |||||||||||||||||
| 25–35 | Compared to UHPC without CTWP, the energy intensity, and CO | 2 | emissions of UHPC with 55% CTWP were reduced by 41.0% and 33.1%, respectively. | 4.190 | |||||||||||||
| DCP50 | 12.5%DCP1 | 0.5 | 33.00 | 102.00 | 337.00 | 0.894 | |||||||||||
| DCP100 | 25%DCP1 | 0.5 | 33.00 | 100.50 | 298.00 | NA | d50: 17.15 μm | 15, 30, 45 | 15 | ||||||||
| - | - | 4.810 | - | [469][470] | Humid hardened concrete waste (HHCW) | Ordinary concrete | Cement (OPC- PI 52.5), HHCW, FA, GGBFS, machine-made sand, river sand, crushed stone, SP. | Multiple wet grinding | d50: 26.5 μm, 5.71 μm, and 2.52 μm | 5, 10, 15[ | |||||||
| 0.790 | 0.5 | 33.00 | 83.00 | 219.00 | 0.581 | ||||||||||||
| [443][444] | Brick powder | 65.240 | 18.080 | 4.250 | 21.700 | 5.100 | 3.400 | 65.000 | 1.400 | 0.300 | 0.550 | 1.500 | 1.050 | ||||
| Periwinkle shell (PS) | |||||||||||||||||
| DCP150 | 37.5%DCP1 | 0.5 | 33.00 | 95.50 | 258.00 | 0.684 | |||||||||||
| Ordinary concrete | |||||||||||||||||
| DCP200 | 50%DCP1 | c | NAC | Control mix | 0.45 | - | 36.80 | - | 1.000 | ||||||||
| RP1 | 10%RP | 0.45 | - | 36.00 | - | 0.980 | |||||||||||
| RP1 | 20%RP | 0.45 | - | 33.50 | - | 0.950 | HHCWS of 2.52 μm at the dosage of 10% | ||||||||||
| 5 | |||||||||||||||||
| [ | |||||||||||||||||
| 1.470 | 2.190 | ||||||||||||||||
| 376 | ] | [ | |||||||||||||||
| RP1 | 30%RP | - | - | 0.340 | - | ||||||||||||
| 0.45 | - | 27.00 | - | 0.930 | [470][471] | Ground recycled masonry aggregate (GR-RMA) | Ordinary concrete | Cement (CEM I 42.5 R OPC), GR-RMA, NA, MRA, natural sand, SP. | NA | Not stated | 25 | 25% GR and 25–50% MRA |
|||||
| Concrete powder | 31.850 | 7.040 | 4.840 | 48.950 | 1.850 | - | -377] | ||||||||||
| 0.780 | - | ||||||||||||||||
| RP2 | 10%RP | 0.45 | - | 35.50 | - | 0.950 | [471][472] | RCP | Ordinary concrete | Cement (CEM I 42.5), RCP, NA, SP. | NA | d50: 22μm | 10, 20, 30, 40, 50 | <10% | |||
| [469][470 | Scallop shell (SLS) | Ordinary concrete | <10 | [377][378] | |||||||||||||
| Manufacturing Industry | |||||||||||||||||
| Red ceramic waste (RCW) | Structural concrete | 20–40 | [378][379] | ||||||||||||||
| Ceramic waste powder (CWP) | Ordinary concrete | 10–20 | [379][380] | ||||||||||||||
| Self-consolidating concrete | 15 | [380][381] | |||||||||||||||
| High-performance concrete | 25–35 | [381][382][382,383] | |||||||||||||||
| Recycled glass powder (RGP) | Ordinary concrete | 10–20 | [383][384][385][386][387][388][389][390][384,385,386,387,388,389,390,391] | ||||||||||||||
| Environmentally friendly concrete | 25 | [391][392][392,393] | |||||||||||||||
| Self-compacting concrete | 24 | [393][394] | |||||||||||||||
| Fly ash (FA) | Ordinary concrete | 30 | [394][395][395,396] | ||||||||||||||
| Self-compacting concrete | 10–55 | [324][396][325,397] | |||||||||||||||
| Pervious concrete | 10–15 | [329][330] | |||||||||||||||
| High-performance concrete | 30 | [397][398][398,399] | |||||||||||||||
| Granulated blast-furnace slag (GGBFS) | Ordinary concrete | <50 | [399][400] | ||||||||||||||
| Recycled aggregate concrete | <20 | [400][401] | |||||||||||||||
| [422][423] | CLBA | Ordinary concrete | 10, 20, 30, 40 | <40 | CO2 released from limestone calcination is 0.37 kg for the control sample (CAC0), 0.33 kg for CAC10, 0.29 kg for CAC20, 0.26 kg for CAC30, and 0.22 kg for CAC40. | ||||||||||||
| [395][396] | FA | Green structural concrete | 20, 40, 60, 80, 98 | <80 | Compared to commercial Grade 45 concrete, the proposed concrete shows a reduction in CO2 emission of around 70%. | ||||||||||||
| [397][398] | High-strength concrete | 30, 40 | 30–40 | The replacement of FA0 with FA30 and FA40 could potentially reduce the carbon footprint by 22.1% and 21.9% per m3 of concrete, respectively. | |||||||||||||
| [423][424] | Ordinary concrete | ] | OPC25 | 20.04025 | 4.198Fly ash was found to be capable of reducing concrete CO2 emissions by 13% to 15% in typical concrete mixes. | 3.365 | 63.058 | 1.930 | 0.092 | 0.748 | 3.276 | 2.653 | [472][473] | RP | Sustainable concrete | ||
| RP2 | 20%RP | 0.45 | - | 32.00 | - | 0.900 | Sewage sludge ash (SSA) | Ordinary concrete | 10 | [408][409] | |||||||
| Waste marble dust (WMD) | Ordinary concrete | <15 | |||||||||||||||
| [423][424] | GGBFS | Ordinary concrete | 40 | Cement (OPC), RP (RCP, RBP), NCA, NFA, FA, GGFBS, air entrainer admixture, water reducer admixture.40 | Replacing 40% of GGBS with Portland cement in 25 or 32 MPa concrete outputs results in a 22% reduction in CO2 emissions. | ||||||||||||
| [403][404] | PPR | Ordinary concrete | 10, 20, 30, 40, 50 | 10–30 | For a compressive strength of 54 MPa at 91 days, the emission was reduced from 564 kg CO2-eq/m3 of concrete for the reference mixture to 473 kg CO2-eq/m3 of concrete (i.e., 16%) for 30% replacement and to 349 kg CO2-eq/m357][358][355,356,357,358,359] | ||||||||||||
| Green concrete | 10–15 | [16][322][16,323] | |||||||||||||||
| Eco-friendly structural foamed concrete | 5 | [341][342] | |||||||||||||||
| NA | d50: RCP: 11.8 μm, RBP: 13.4 μm | 20 | HHCW | 29.689 | 7.948 | 2.453 | 31.713 | 2.728 | 0.842RBP can provide equivalent |
1.078 | 0.685 | ||||||
| RP2 | strength and even better durability. | ||||||||||||||||
| 21.986 | |||||||||||||||||
| 30%RP | 0.45 | - | 27.50 | - | 0.850 | [473][474] | WP | WP concrete | Cement (OPC), WP (mixture of waste concrete and bricks), NA, sand. | [NA | 470d50: 12.54 μm | 15, 30, 45 | ][471] | GR-RMA | 60.00015 | ||
| 19.000 | 6.000 | - | - | - | - | - | - | ||||||||||
| RP3 | 10%RP | 0.45 | - | 34.00 | - | 0.930 | [474][475] | RP | Reactive powder concrete | Cement (PO 42.5), RP (abandoned clay bricks and cement solids), SF, SP. | NA | d50: 31.4 μm | 5, 10, 15, 25 | 10 | |||
| [472][473] | OPC | 21.300 | 3.200 | 2.900 | 64.300 | 2.100 | 0.260 | 0.420 | 3.100 | 1.350 | [475][476] | Waste concrete powder (WCP) | Self-consolidating concrete | Cement (OPC), WCP, GGBFS, NCA, NFA, SP. | NA | d50: 90 μm | 15, 30, 45 |
| RCP | 51.000 | 10.130 | 5.360 | 26.310 | 1.380 | 1.230 | |||||||||||
| RP3 | 20%RP | 0.45 | - | 31.50 | - | 0.850 | 15 | ||||||||||
| 1.780 | 1.940 | 9.900 | |||||||||||||||
| RP3 | 30%RP | 0.45 | - | 27.50 | - | 0.780 | [476][477] | RP | Ordinary concrete | RBPCement (OPC), RP (80% fired brick and 20% waste concrete), NA, sand, water reducer. | |||||||
| [464][465 | NA | ] | <75 μm | 15, 30, 45 | 69.870 | 20.980 | <30% | ||||||||||
| 3.610 | NAC | Control mix | 0.400 | 0.390 | 0.56 | 1.0–1.5% | 46.600.590 | 2.420 | 0.330 | 0.980 | [477][478] | Waste brick powder (WBP) | Ordinary concrete | ||||
| 269.83 | 1.000 | Cement (type II OPC), WBP, NCA, sand. | NA | d50: 45 μm | 10, 15, 20, 25, 30, 40 | <20% | |||||||||||
| [448][449] | OPC | 19.900 | 4.420 | 3.560 | 64.900 | 0.660 | 0.080 | 0.790 | 2.670 | - | |||||||
| N10/0 | 10%GRC | 0.58 | 1.0–1.5% | 37.80 | 249.65 | 0.925 | [478][479] | Cement kiln dust (CKD) | Ordinary concrete | Cement (ASTM C 150 Type I and Type V), CKD, NCA, sand. | RP | 57.010NA | not stated | 10.930 | 3.450 | 21.3005, 10, 15 | 5 |
| 1.820 | 1.580 | 2.220 | 1.170 | - | |||||||||||||
| N25/0 | 25%GRC | 0.60 | 1.0–1.5% | 27.70 | 218.43 | 0.810 | [479][480] | CKD | Self-consolidating Concrete | Cement (OPC Type I), CKD, NCA, sand, SP. | NA | not stated | [487] | 10, 20, 30, 40 | [488] | 20 | |
| R0/50 | OPC | 0%GRC, 50% RA-CDW | 0.59 | 1.0–1.5% | 34.80 | 267.10 | 0.990 | [448][449] | CKDRP | Sustainable recycled concrete | Cement (PO42.5), RP, FA, NCA, NFA, river sand, SP. | NA | <45 μm | 15, 30 | 15 | ||
| 11.690 | 3.250 | 2.400 | 44.900 | 0.800 | 0.290 | 0.500 | Sustainable foamed concrete | 5 | [344][345] | ||||||||
| 0.000 | Olive waste ash (OWA) | Ordinary concrete | 5 | [359][360] | |||||||||||||
| R10/50 | 10%GRC, 50% RA-CDW | 0.61 | 36.000 | 1.0–1.5% | 32.80 | 246.94 | 0.915 | [480][481] | Ceramic (fired clay-based) fraction of CDW | Structural concrete | Cement (CEM I 42.5 R), ceramic (fired clay-based) fraction of CDW, NCA, RA, sand, SP. | ||||||
| R25/50 | 25%GRC, 50% RA-CDW | 0.63 | NA | not stated | 1.0–1.5% | 23.30 | 216.7025, 50 | 25 | |||||||||
| 0.803 | [481][482] | CKD | High performance self-compacting concrete | Cement (OPC), CKD, NCA, mineral sand, SP. | NA | <50 μm | 10, 20, 30 | <10% | |||||||||
| [467][468] c | RCP0 | Control mix | 0.16 | 41.64 | 100.00 | 502.63 | 1.000 | [482][483] | Burnt clay and CKD | Blended concrete | Cement (OPC), burnt clay and CKD, NCA, NFA. | NA | <75 μm | 10, 20, 30, 40 | <20%CKD | ||
| RCP15 | 15%RCP | 0.16 | 40.06 | 82.80 | 501.75 | 0.998 | [483][484] | CKD | Ordinary concrete | Cement (cement of Indian Standards (IS) mark 43 grade), CKD, NCA, NFA. | Bacterial treatment | not stated | 5, 10, 15 | 10% | |||
| [ | |||||||||||||||||
| RCP30 | 30%RCP | 0.16 | 39.08 | 96.10 | 500.86 | 0.996 | 484][485] | Clay brick powder (CBP) | Ordinary concrete | Cement (OPC), CBP (Recycled construction waste), natural sand. | NA | d50: 300 μm, 100 μm, 60 μm and 40 μm | 10, 20, 25, 30 | 10% | |||
| [485 | |||||||||||||||||
| RCP45 | 45%RCP | 0.16 | 37.13 | 88.30 | 499.27 | 0.993 | ][486] | Construction waste composite powder | Small-scale prefabricated concrete | Cement (42.5 OPC), CWBP (building demolition waste), NCA, sand. | NA | d50: 8–16 μm | |||||
| [468 | 20, 30, 40 | ][469 | 30 | ||||||||||||||
| ] | RAPC-0–0 | Control mix | 0.49 | 0.14 | 39.04 | - | [486][487] | GRC | Ordinary concrete | Cement (CEM I 42.5 R), GRC, NCA, MRA. | NA | <147 μm | 10, 25 | 25 | |||
| 1.000 | |||||||||||||||||
| RAPC-0–15 | 15%RP | 0.49 | 0.16 | 40.12 | - | 0.850 | [487][488] | CKD | Green concrete | Cement (OPC Type II), CKD, FA, river sand, NCA, SP. | NA | <45 μm | 10, 15, 20, 30, 40 | <20% | |||
| RAPC-0–30 | 30%RP | 0.49 | 0.17 | 35.45 | - | 0.710 | [488][489] | CBP | Ordinary concrete | Cement (OPC), CBP (mainly, bricks and tiles), NA, recycled gravel. | NA | <63 μm | 25 | ||||
| RAPC-0–45 | 25 | ||||||||||||||||
2.4.1. CO2 Reduction by the Partial Replacement of Portland Cement with CDW
Studies conducted regarding CO2| 45%RP | ||||||||||
| 0.49 | ||||||||||
| 0.16 | ||||||||||
| 30.27 | ||||||||||
| - | ||||||||||
| 0.560 | ||||||||||
| RAPC-30–0 | ||||||||||
| 30%RA + 0%RP | ||||||||||
| 0.49 | ||||||||||
| 0.14 | ||||||||||
| 41.17 | ||||||||||
| - | ||||||||||
| 1.000 | ||||||||||
| High-strength concrete | ||||||||||
| 5 | ||||||||||
| RAPC-30–15 | 30%RA + 15%RP | 0.49 | 0.16 | 43.29 | - | 0.850 | ||||
| RAPC-30–30 | 30%RA + 30%RP | 0.49 | 0.17 | 37.45 | - | 0.700 | ||||
| RAPC-30–45 | 30%RA + 30%RP | 0.49 | 0.16 | 31.32 | - | 0.560 | ||||
| RAPC-50–0 | 50%RA + 0%RP | 0.49 | 0.14 | 36.44 | - | 0.990 | ||||
| RAPC-50–15 | 50%RA + 15%RP | 0.49 | 0.16 | 37.28 | - | 0.850 | ||||
| RAPC-50–30 | 50%RA + 30%RP | 0.49 | 0.17 | 33.56 | - | 0.700 | ||||
| RAPC-50–45 | 50%RA + 45%RP | 0.49 | 0.16 | 29.56 | - | 0.550 | ||||
| RAPC–100–0 | 100%RA + 0%RP | 0.49 | 0.14 | 33.26 | - | 0.990 | ||||
| Steel slag (SS) | ||||||||||
| RAPC-100–15 | 100%RA + 15%RP | 0.49 | 0.16 | 35.18 | - | 0.840 | ||||
| Ordinary concrete | ||||||||||
| RAPC-100–30 | 100%RA + 30%RP | 0.49 | 0.17 | 28.36 | - | 0.690 | ||||
| RAPC-100–45 | 100%RA + 30%RP | 0.49 | 0.16 | 22.79 | - | 0.550 | ||||
| [470][471] | CC | Control mix | 0.45 | 6.20 | 51.2 | 407.00 | 1.000 | |||
| C25 | 0%CDW + 25% MRA | 0.45 | 6.20 | 51.7 | 399.00 | 0.980 | ||||
| C50 | 0%CDW + 50% MRA | 0.45 | 6.20 | 51.1 | 351.00 | 0.862 | ||||
| R25/0 | 25%CDW | 0.45 | 6.20 | 46.1 | 335.00 | 0.823 | ||||
| 20 | ||||||||||
| R25/25 | 25%CDW + 25% MRA | 0.45 | 6.20 | 45.7 | 327.00 | 0.803 | ||||
| R25/R50 | 50%CDW + 50% MRA | 0.45 | 6.20 | |||||||
| [ | ||||||||||
| 401 | ||||||||||
| ] | ||||||||||
| [ | ||||||||||
| 402 | ||||||||||
| ] | ||||||||||
| 41.2 | 319.00 | 0.784 | ||||||||
| [471][472] | RCP0 | Control mix | 0.55 | 3.00 | 51.60 | 333.00 | 1.000 | |||
| RCP10 | 10%RCP | 0.55 | 3.00 | 41.30 | 304.00 | 0.913 | High-early-strength concrete | 30 | [402 | |
| RCP20 | 20%RCP | 0.55 | 3.00 | 31.70 | ][403] | |||||
| 275.00 | 0.826 | |||||||||
| RCP30 | 30%RCP | 0.55 | 3.00 | 22.80 | 246.00 | 0.739 | ||||
| RCP40 | 40%RCP | 0.55 | 3.00 | 13.60 | 217.00 | 0.652 | ||||
| RCP50 | 50%RCP | 0.55 | 3.00 | 10.00 | 188.00 | 0.565 | Silica fume (SF) | Ordinary concrete | 10 | [357][358] |
| Self-compacting concrete | 10 | |||||||||
| [448][449] d | Control | Control mix | 0.36 | 2.16% | 877.30 | 367.50 | 1.000 | |||
| RP1 | 15%RP | 0.36 | 2.84% | 613.92 | 325.00 | 0.884 | ||||
| RP2 | 30%RP | 0.36 | 3.52% | 786.23 | 278.00 | 0.756 | ||||
| RP3 | 15%RP + 15%FA | 0.36 | 2.50% | 1298.73 | 275.60 | 0.750 | ||||
| [487][488] | Ctrl-W37 | Control mix | 0.37 | 0.33 | 53.41 | 510.77 | 1.000 | |||
| C5W37 | 5%CKD | 0.37 | 0.33 | 55.47 | 487.57 | 0.955 | ||||
| C10W37 | 10%CKD | 0.37 | 0.33 | 52.13 | 464.36 | 0.909 | ||||
| C15W37 | 15%CKD | 0.37 | 0.45 | 47.45 | 441.24 | 0.864 | ||||
| C20W37 | 20%CKD | 0.37 | 0.54 | 41.42 | 418.10 | 0.819 | ||||
| C30W37 | 30%CKD | 0.37 | 0.67 | 34.90 | 371.79 | 0.728 | ||||
| C40W37 | 40%CKD | 0.37 | 1.63 | 28.09 | 326.07 | 0.638 | [324] | |||
| Ctrl-W40 | Control mix | [325] | ||||||||
| 0.40 | 0.00 | 52.23 | 476.71 | 1.000 | Recycled aggregate concrete | 10 | [400][401] | |||
| C5W40 | 5%CKD | 0.40 | 0.00 | 49.52 | 457.94 | 0.961 | Porcelain Tile Polishing Residue (PPR) | Ordinary concrete | 10–40 | [403][404] |
| Self-compacting concrete | ||||||||||
| C10W40 | 10%CKD | 0.40 | 0.00 | 43.24 | 433.78 | 0.910 | 25 | [404][405] | ||
| C15W40 | 15%CKD | 0.40 | 0.00 | 37.97 | 412.32 | 0.865 | Electric Arc Furnace Dust (EAFD) | Ordinary concrete | 10 | [405][406] |
| C20W40 | 20%CKD | 0.40 | 0.00 | 36.93 | 390.85 | 0.820 | [317][409][410][318,410,411] | |||
| High-strength concrete | 15 | [411][412] | ||||||||
| 3 | of concrete (i.e., 38%) for 50% addition. | |||||||||
| [404][405] | Self-compacting concrete | 10, 20, 30 | <20 | For a compressive strength of 70 MPa, the incorporation of PPR would reduce the emission of CO2-eq/m3 of concrete by up to 17% when incorporating 127 kg of the residue per m3 of concrete. | Red mud (RM) | Ordinary concrete | 12 | [406][407] | ||
| C30W40 | 30%CKD | 0.40 | 0.33 | 34.94 | 348.16 | 0.730 | Sustainable concrete | 10–15 | [407][408] | |
| C40W40 | 40%CKD | 0.40 | 0.67 | 28.79 | 305.48 | 0.641 | Titanium dioxide (TiO2 | |||
| Ctrl-W45 | Control mix | 0.45 | 0.00 | 50.14 | 430.36 | 1.000 | ) nanoparticles | |||
| C5W45 | 5%CKD | 0.45 | 0.00 | 46.93 | 411.29 | 0.956 | Blended cement concrete | |||
| C10W45 | 10%CKD | 0.45 | 0.00 | 44.76 | 392.20 | 0.911 | 3 | [412][413] | ||
| C15W45 | 15%CKD | 0.45 | 0.00 | 40.75 | 373.13 | 0.867 | Coal bottom ash (CBA) | Sustainable concrete | ||
| C20W45 | 20%CKD | 15 | [413][ | 0.45414] | ||||||
| 0.00 | 37.53 | 354.05 | 0.823 | Copper Slag (CS) | Ordinary concrete | 10 | ||||
| C30W45 | 30%CKD | 0.40 | [395][414][396,415] | |||||||
| 0.00 | 34.79 | 315.89 | 0.734 | Foundry sand waste (FSW) | Ordinary concrete | <30 | [415][416][416,417] | |||
| Polyvinyl chloride (PVC) waste powder (WP) | Green concrete | 15–20 | [294][296] | |||||||
| Others | ||||||||||
| Limestone powder (LP) | Self-consolidating concrete | 55 | [396][397] | |||||||
| Ultra-high-performance concrete | 54 | [417][418] | ||||||||
| Metakaolin (MK) | High-performance concrete | 10 | [398][399] | |||||||
| Volcanic ash (VA) | Ordinary concrete | 10–15 | [418][419] | |||||||
| Crushed rock dust (CRD) | Ordinary concrete | 20 | [292][294] | |||||||
| Municipal solid waste incineration ash (MSWI) | Ordinary concrete | <12 | [295][419][297,420] | |||||||
CO2 Reduction through the Partial Replacement of Portland Cement with MA
CO2 reduction by the partial replacement of Portland cement with MAs is reviewed for two cases: first for binary blended cements in Table 5, and second for ternary blended cements in Table 6.Table 5.
CO
2
reduction through the partial replacement of Portland cement with AM (binary blended cements).
| Reference | MA | Mix Type | Amounts of Substitution (wt.%) | Optimum Substitution (wt.%) | Results for CO2 Emmisions | |
|---|---|---|---|---|---|---|
| [420][421] | Biochar rice husk (BRH) | Ordinary concrete | 5, 10, 15, 20 | Not stated | Global warming values (kg CO2eq) for BRH0%, BRH5%, BRH10%, BRH15% and BRH20% are 2.51 × 10 | |
| [ | ||||||
| 424 | ||||||
| ] | ||||||
| [ | ||||||
| 425 | ||||||
| ] | ||||||
| SF | ||||||
| High-strength concrete | ||||||
| 8, 10, 12 | ||||||
| 12 | The climate change index for reference concrete is 534.26 kg CO | 2 | eq. Values for HSC-SF8, HSC-SF10, and HSC-SF12 are 520.75, 495.11 and 453.15, respectively. | |||
| [424][425 | ||||||
| C40W45 | ||||||
| 40%CKD | ||||||
| 0.40 | ||||||
| 0.33 | ||||||
| 28.59 | ||||||
| 277.96 | ||||||
| 0.646 | ||||||
| C5F15W37 | ||||||
| 5%CKD + 15%FA | ||||||
| 0.37 | ||||||
| 0.33 | ||||||
| 55.93 | ||||||
| 419.60 | ||||||
| 0.822 | ||||||
| C10F15W37 | ||||||
| 10%CKD + 15%FA | ||||||
| 0.37 | ||||||
| 0.33 | ||||||
| 48.64 | ||||||
| 396.52 | ||||||
| 0.776 | ||||||
| C5F15W40 | ||||||
| 5%CKD + 10%FA | ||||||
| 0.40 | ||||||
| 0.00 | ||||||
| 45.69 | ||||||
| 392.52 | ||||||
| 0.823 | ||||||
| C10F15W40 | ||||||
| 10%CKD + 15%FA | ||||||
| 0.40 | 0.00 | 46.44 | 371.28 | 0.779 | ||
| C5F15W45 | 5%CKD + 10%FA | 0.45 | 0.00 | 44.19 | 355.83 | 0.827 |
| C10F15W45 | 10%CKD + 15%FA | 0.45 | 0.00 | 40.03 | 336.44 | 0.782 |
a Water–binder ratio. b Superplasticizer; if % is not stated, the values are in kg/m3. c 28 day compressive stresses are approximately derived from the figure. d Static yield stresses (Pa) are given in the study.
In their study, Qian et al. [463][464] examined a viable approach to the production of environmentally friendly ultra-high-performance concrete (UHPC) through the integration of recycled concrete waste coarse aggregate material (RCWCM). By subjecting RCWCM to a heating treatment process, they produced DCP. Subsequently, DCP was employed in a progressive manner to substitute the Portland cement content, thereby being incorporated into the formulation of UHPC utilizing the modified Andreasen and Andersen particle packing model. The findings indicate that the substitution of up to 25% Portland cement with DCP does not significantly affect the compressive strength variation of UHPC. Moreover, the researchers utilized the EN ISO 14040 and EN ISO standards to evaluate the environmental impact of UHPC by employing the carbon footprint metric. To establish the sustainability and environmental cleanliness of the UHPC, this study undertook calculations to determine CO2 emissions per unit of green UHPC with varying DCP levels. Additionally, the ratio of CO2 emissions to compressive strength per unit of green UHPC was also evaluated. From the results, it can be noticed that the inclusion of DCP yields advantageous outcomes in enhancing the performance of UHPC from a sustainability perspective.
The objective of the study carried out by Kim and Jang [443][444] was to examine the feasibility of closed-loop recycling for construction waste. Specifically, the focus was on examining the impact of utilizing concrete powder, which is a byproduct of producing recycled aggregates, on the fresh and hardened mechanical properties of concrete. The authors assert that concretes produced using recycled materials such as RCA, RFA, and RP exhibit a lower cost compared to natural coarse aggregate (NAC). However, it is important to note that these recycled concretes also have reduced compressive strength. Additionally, it was asserted that the utilization of RP as a substitute for Portland cement yields environmental advantages, including reductions in CO2 emissions, the preservation of natural resources, and the mitigation of landfill usage.
Cantero et al. [464][465] examined the cumulative impact of using ground recycled concrete (GRC) as a Portland cement replacement along with the use of mixed recycled construction and demolition waste aggregate (RA-CDW) in the context of structural concrete. The mechanical performance of concrete mixes with GRC and recycled aggregate from CDW (RA-CDW) was shown to be inferior compared to mixes made solely with natural aggregate and cement. However, it is worth noting that the difference in performance was relatively smaller when considering the corresponding replacement ratios. The authors did not consider the emissions associated with manufacturing and transportation when assessing the environmental impact of the mixtures in terms of CO2 emissions from materials. These emissions were considered smaller than those created during material manufacturing. In accordance with the provided statistics, the implementation of GRC resulted in a reduction in CO2 emissions by 7.5% in N10/0, 18.7% in N25/0, 8.5% in R10/50, and 19.7% in R25/50. The utilization of GRC, in conjunction with RA-CDW, has been found to augment the environmental efficacy of concrete. When the replacement rate was set at 10%, the amount of CO2 released during the manufacturing process of concrete decreased by 8.5% compared to concrete produced with OPC and 100% natural aggregates (NA). Similarly, when the replacement rate was increased to 25%, the greenhouse gas emissions associated with GRC decreased by 19.7% compared to OPC-based concrete with 100% NA.
The study conducted by He et al. [467][468] aimed to evaluate the influence mechanism of RCP on the multi-scale properties of UHPC mixtures. The findings of the study revealed that the UHPC combination with 30% RCP exhibited a comparatively reduced strain in early-age autogenous shrinkage, along with the highest mechanical characteristics. The reference parameters used by the authors to assess UHPC’s positive environmental impact included the mixture’s total carbon emissions and non-renewable energy consumption (NREC). The study demonstrates that there is a decrease in the NREC per cubic meter of UHPC mixture when the RCP substitution ratio increases. In parallel, it can be observed that the augmentation in the substitution proportion of RCP leads to a corresponding reduction in the carbon emissions per unit volume of UHPC mixture.
The objective of the study of Wu et al. [468][469] was to examine the characteristics of pore structure, carbonation, and chloride ion permeability in recycled aggregate-powder concrete (RAPC). The findings of the study indicate that there is a positive correlation between the replacement rate of recycled aggregate (RA) and both the carbonation depth and chloride ion permeability of RAPC. The research indicates that the inclusion of 15% RP resulted in the enhanced performance of RAC. This addition has effectively addressed the issue of by-products generated during the manufacturing of RA, leading to cost reduction and a reduction in the adverse environmental effects associated with RAC production.
The durability of a concrete mixture containing ground recycled masonry aggregate (GR-RMA) as a partial replacement for cement and coarse mixed recycled aggregate (MRA), both obtained from CDW, was examined by Cantero et al. [470][471]. The investigation involved the indirect characterization of pore system permeability by utilizing important indicators of water transport. Based on the results obtained from the defined scenario, it was determined that the optimal combinations of mechanical efficiency and durability were observed in mixes with a 25% GR content as a replacement for Portland cement. Additionally, it was found that the mixes with the highest environmental benefits in terms of reducing CO2 emissions were those that included both 25% GR and 25% to 50% MRA.
In the study done by Pešta et al. [472][473], the researchers evaluated the environmental viewpoints pertaining to the utilization of RCP as a substitute for Portland cement. The findings from the assessment of mechanical properties indicate that RCP exhibits favorable characteristics as a substitute for Portland cement, particularly in scenarios with a low degree of replacement. Furthermore, the findings of the environmental assessment provide confirmation that the implementation of RCP resulted in a decrease in the adverse effects of climate change, as well as potential effects in other related domains.
Singh et al. [448][449] examined the practical application of recycled fines (RFs), namely RFA and RP, in the context of recycled concrete. The investigation focused on evaluating the fresh qualities (empirical and rheological) of the recycled concrete. The findings indicated that the decrease in slump was more pronounced in the series with RFA compared to RP. According to the authors, the inclusion of RF in concrete mixtures not only enhances material performance but also presents notable environmental advantages, specifically in mitigating carbon emissions linked to the production of concrete.
Bagheri et al. [487][488] utilized varying quantities of CKD, a waste material, and FA, a pozzolanic material, as replacements for Portland cement, both alone and in combination. The comparison between the Taguchi technique and experimental outcomes for the purpose of picking the most advantageous mixture designs revealed that the Taguchi approach demonstrated appropriate selections within the range of optimal experimental results taking into consideration the initial parameters. Furthermore, the values for the cost and CO2 emission factors of each plan were determined by considering the CO2 production cost associated with each material and the corresponding size of said material inside the relevant plan. The observed decrease in cost of 23% resulting from the substitution of Portland cement with cement additions, alongside the concurrent reduction in volume within the C40W45 mixture, was found to be statistically significant. Additionally, it is worth noting that the CO2 emission factor associated with the Ctrl-W37 value (510.8 kg/m3) exhibited a reduction of almost 50% when considering the C40F0W45 mixture (278 kg/m3).
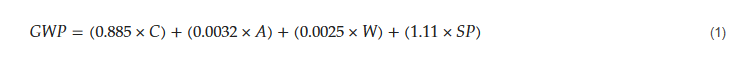 where C is the cement content of concrete (kg/m3), A is the aggregate content (kg/m3), W is the water content (kg/m3), and SP is the superplasticizer content (kg/m3).
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 display the collected data on the GWP of the produced concrete, as well as the ratio of GWP to compressive strength per unit of the concrete, for various levels of CDW content. The second measure indicated above corresponds to the quantity of GWP per unit of strength. A higher GWP/compressive strength ratio in the produced concrete indicates a bigger quantity of carbon dioxide generated during the production of concrete, provided that the compressive strength remains constant. Based on the results depicted in the figures, it is evident that the inclusion of CDW has a positive impact on enhancing the performance of concrete from a sustainability perspective.
where C is the cement content of concrete (kg/m3), A is the aggregate content (kg/m3), W is the water content (kg/m3), and SP is the superplasticizer content (kg/m3).
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 display the collected data on the GWP of the produced concrete, as well as the ratio of GWP to compressive strength per unit of the concrete, for various levels of CDW content. The second measure indicated above corresponds to the quantity of GWP per unit of strength. A higher GWP/compressive strength ratio in the produced concrete indicates a bigger quantity of carbon dioxide generated during the production of concrete, provided that the compressive strength remains constant. Based on the results depicted in the figures, it is evident that the inclusion of CDW has a positive impact on enhancing the performance of concrete from a sustainability perspective.
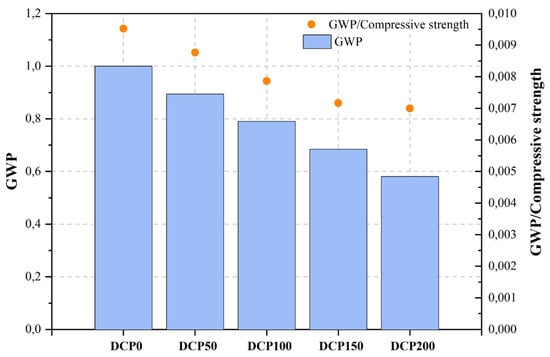
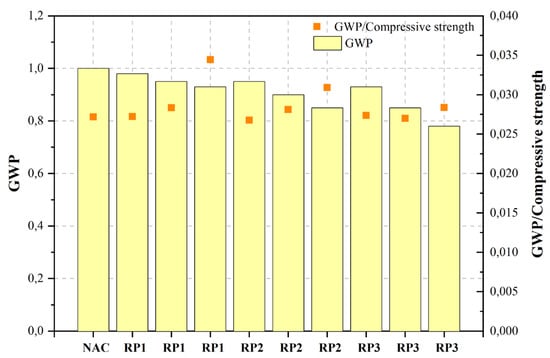
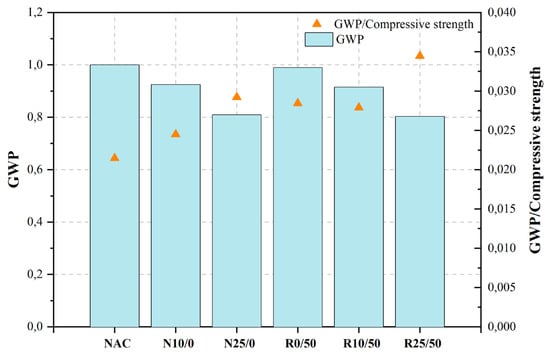
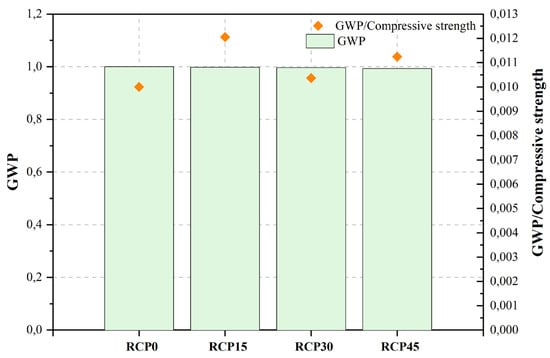
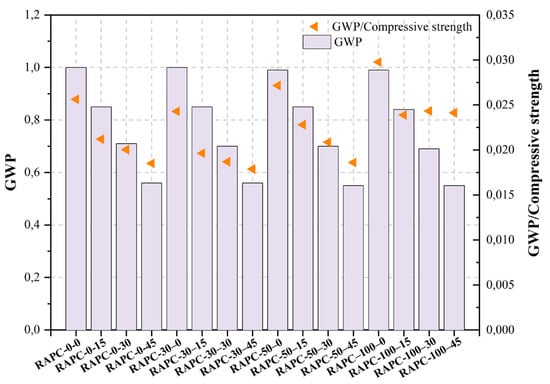
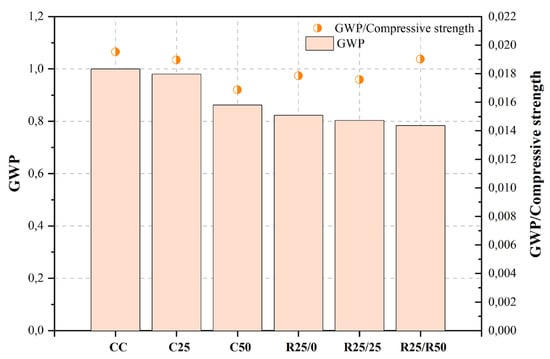
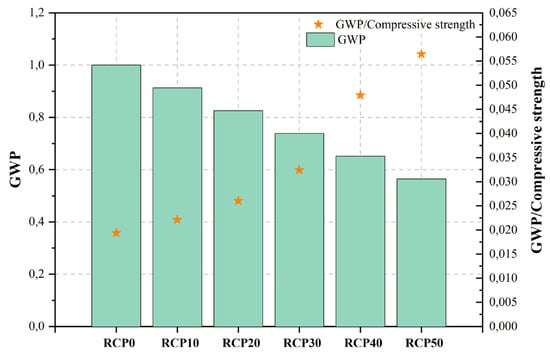
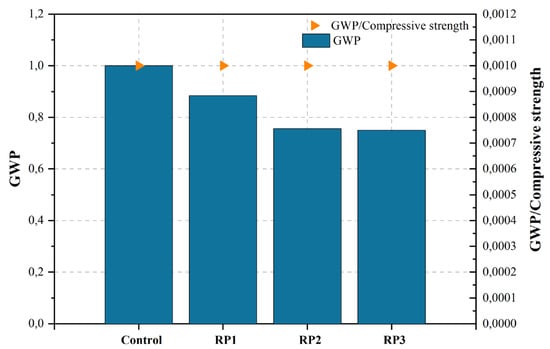
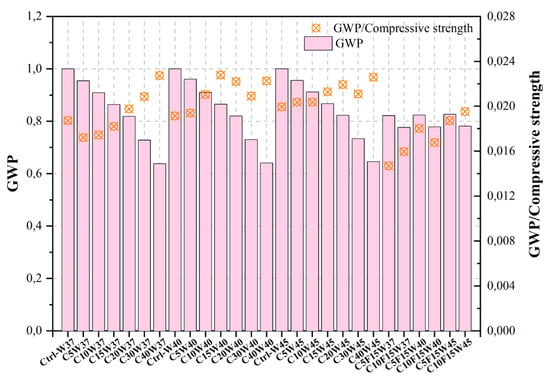 When comparing the identical strength conditions, the produced concrete including DCP [463][464], RP [462][463], GRC [464][465], RCP [467][471][468,472], RAPC [468][469], GR-RMA [470][471], RF [448][449], and CKD [487][488] exhibits a lower CO2 emission per unit volume compared to the reference sample. This indicates a higher efficiency in utilizing Portland cement in the produced concrete. In addition, the increase in all types of CDW contents leads to a significant reduction in carbon dioxide emissions during the production of the concrete.
When comparing the identical strength conditions, the produced concrete including DCP [463][464], RP [462][463], GRC [464][465], RCP [467][471][468,472], RAPC [468][469], GR-RMA [470][471], RF [448][449], and CKD [487][488] exhibits a lower CO2 emission per unit volume compared to the reference sample. This indicates a higher efficiency in utilizing Portland cement in the produced concrete. In addition, the increase in all types of CDW contents leads to a significant reduction in carbon dioxide emissions during the production of the concrete.
2.4.2. Evaluation of CO2 Emissions with Respect to Compressive Strength
For the studies presented in Table 9, the global warming potential (GWP) was calculated (Equation (12)) using the environmental parameter presented by Khodabakhshian et al. [489][490]. The GWP was formulated to quantify the alteration in the greenhouse effect resulting from human-caused emissions and absorptions.