Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by João Martins Gama and Version 2 by Rita Xu.
Leiomyosarcomas (LMSs) are malignant neoplasms of soft muscle differentiation that can be classified into five distinct groups according to site-related origin: intra-abdominal, subcutaneous or deep soft tissue of the limbs, cutaneous, external genitalia, and vascular.
- leiomyosarcoma
- inferior vena cava leiomyosarcoma
- vascular
1. Introduction
Leiomyosarcoma (LMS) is a malignant neoplasm of soft muscle differentiation [1]. Although uncommon, it is one of the most common sarcomas. LMSs of the soft tissue can be grouped into five distinctive categories according to site-related origin: intra-abdominal, subcutaneous or deep soft tissue of the limbs, cutaneous, external genitalia, and vascular. The distinction is important since there are clinical differences between these subgroups [2]. Intra-abdominal and deep soft tissue are the most common subgroups, and the least common is the vascular LMS group. Although rare, LMS is the most common malignant tumor involving the vascular system [3]. Vascular LMS originates in the walls of medium-sized or large blood vessels, usually from the inferior vena cava. This distinction is fundamental since it reflects distinct biological origins, molecular changes, and prognoses.
Inferior vena cava LMS was first described by Leopold Perl and Rudolph Virchow in 1871 [4]. Since then, many discoveries have been made and a clearer image of this interesting entity has emerged.
2. Epidemiology
It is estimated that LMS of the soft tissue represents up to 15% of all soft tissue sarcomas [5]. LMS of vascular origin is rare, and most of the knowledge gathered so far comes from isolated case reports or small series. It is estimated that vascular LMS represents 5% of soft tissue LMS, and half arise from the inferior vena cava or veins of the lower limbs. Leiomyosarcomas are five times more common in veins than arteries [6]. Autopsy-based studies estimated an incidence of 1/7000-34,000 autopsies [7]. Currently, less than 400 cases are described in the literature. Older adults are usually the most affected, with a median age at diagnosis of 56 years (range: 34–75) [8], but interestingly, more than three-quarters of all vena cava leiomyosarcomas occur in women [8][9][8,9]. The hormonal influence on growth and proliferation of smooth muscle tissue might explain this. LMS is the most common malignancy in the vascular system in adults, and albeit rare, some cases have been reported in the pediatric age [10]. The distribution of vascular leiomyosarcomas is grossly inversely proportional to the pressure in the vascular bed [7][11][7,11]. Vascular leiomyosarcomas are more common in large veins, where the pressure is lower [12]; the most common location is in the inferior vena cava, followed by other large veins. Less commonly, vascular LMSs can arise in the pulmonary artery and in large systemic arteries [6][11][6,11]. In 1973, Kevorkian and Cento [11] conducted an extensive review of all of the cases of vascular leiomyosarcomas published in the literature, and out of a total of 86 patients, 33 were found in the inferior vena cava, 35 in other large veins, 10 in the pulmonary artery, and 8 cases in large systemic arteries. LMS arising in an arteriovenous fistula has also been reported [13].3. Clinical Presentation
The clinical symptoms are diverse, non-specific, dependent on the location of the tumor, the growth rate, and the development of collateral blood flow [6]. When leiomyosarcomas develop in the superior vena cava segment, symptoms manifest as Budd–Chiari syndrome with hepatomegaly, jaundice, ascites, and nausea. In the middle segment, the symptoms are associated with abdominal discomfort and sometimes related to vascular compromise (e.g., edema of the lower limbs) [14]. In the inferior segment, the symptoms appear relatively late (e.g., edema, nausea, and back pain) [15][16][18,19]. Rarer presenting symptoms are recurrent pulmonary embolisms and metastases. LMSs may remain asymptomatic and are often incidentally diagnosed [11].4. Etiology and Pathogenesis
Not much is known about the predisposing factors and etiology of vascular LMS, and the experience gathered from leiomyosarcomas from other sites is not always directly applicable to the soft tissue and vascular counterpart [7]. Leiomyosarcomas have a complex karyotype with complex numeric and structural anomalies, and multiple genes have been implicated in its pathogenesis [17][20], with significant mutational heterogeneity and frequent copy number variations with no characteristic chromosomal rearrangements [18][21]. In a study by Chudasama et al., chromothripsis was reported in 35% of LMSs. Losses of chromosome regions encoding for tumor suppressor genes such as TP53, PTEN, CDH1, and MYOCD have been reported [18][19][21,22], as well as genes involved in DNA homologous recombination repair (BRCA2 and ATM are a frequent target of deletions). Other recurrently mutated genes are chromatin modifiers (RBL2, DNMT3A, and KAT6B), cytokine receptors (ALK, FGFR2, FLT3, and LIFR), and transcriptional regulators (PAX3, FOXO1, CDX2, and SUFU) [18][21]. Alternative telomere lengthening, with alterations in telomere maintenance genes such as ATRX, RBL2, and SP100, were identified in 78% of leiomyosarcomas [18][21]. In the same study, anomalies in the Retinoblastoma–cyclin D1 pathway and TP53 were found in almost every single case of LMS.5. Imaging Diagnosis
Radiology is essential for the diagnosis, and since the symptoms are rather unspecific, the clinical differential diagnosis is broad, encompassing entities such as liposarcomas and lymphomas [20][23]. Invariably, the clinician will order an imaging exam such as ultrasonography (US), computerized tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET). US is rather unspecific [21][24], so CT is usually the method of choice for the first approach, enabling imaging-guided biopsy [22][23][25,26]. MRI has no specific signal; it is usually hypointense in T1 and intermediate in T2 but may be helpful in assessing necrosis [24][27]. MRI has a higher soft tissue resolution, allowing for a better definition of the vascular structures involved, and does not expose the patients to radiation [25][28]. MRI can also be combined with angiography for better local tumor evaluation. Contrast-enhanced cavography MRI can be used to differentiate an intraluminal mass from a thrombus and will also allow for the determination of the degree of obstruction and the development of collateral circulation [26][29]. PET has been proposed as a valuable indicator of tumor grade. A study by Punt et al. has demonstrated a correlation between a higher standard uptake value (SUVmax) and higher histological grade and tumor size [27][30]. In addition, it is a useful tool for assessing distant metastases [25][28]. Therefore, PET is valuable for a pre-operative decision. Despite the value of radiology, pathology is fundamental and the gold standard in assessing LMS [28][31].6. Gross and Histologic Features
On gross examination, the majority are extraluminal (76%), with minimal or even absent luminal growth. Tumor size can range from 2 to 30 cm [8][9][8,9]. Histologically, the cells are elongated in shape, with abundant and eosinophilic cytoplasm, centrally placed nuclei, and blunt-ended edges, sometimes called “cigar-shaped”. Architecturally, the cells are arranged in fascicles [1]. Cytoplasmatic and perinuclear haloes are frequently observed [29][15]. An example can be seen in Figure 1.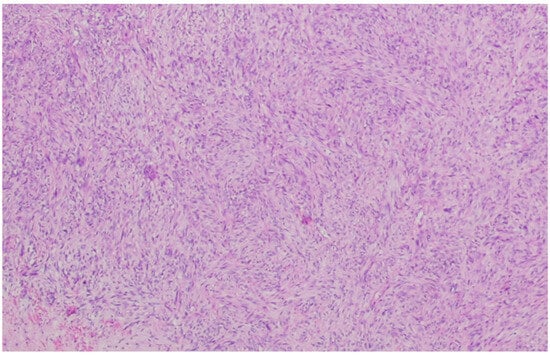
Figure 1. Leiomyosarcoma composed of cells with a fascicular architecture. The tumor cells are elongated with an eosinophilic cytoplasm. The cells, although atypical, resemble normal smooth cells, indicating mild atypia.
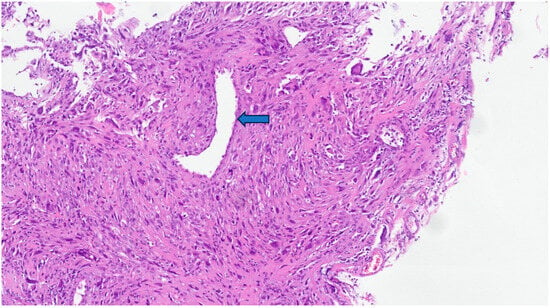
Figure 2. In this leiomyosarcoma, there is a pronounced atypia with pseudoinclusions and pleomorphic, hyperchromatic, and bizarre nuclei. The preserved vascular lumen can be recognized in the central region of the photo (arrow).
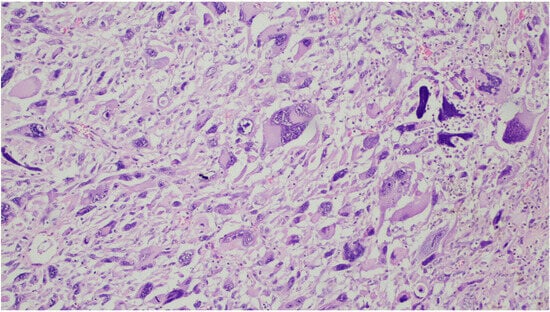
Figure 3. In this tumor, the cells are markedly pleomorphic with multinucleation.
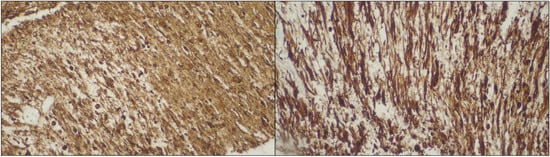
Figure 4. Diffuse staining for α-SMA (left side) and desmin (right side). Abbreviations: α-SMA—α-smooth muscle actin.
