The global energy production is currently characterized by an unprecedented shift to renewable energy sources (RES) and their technologies. However, the local and the environmental benefits of such RES-based technologies show a wide variety of technological maturity, with a common mismatch to local RES stocks and actual utilization levels of RES exploitation. Among contemporary sources of energy production, hydrogen can be characterized as an efficient and clean fuel. The role of hydrogen as a clean energy source is a promising but also a contentious issue. This multifaceted role of hydrogen utilization for clean energy production is literature-reviewed and technology-conveyed.
1. Introduction
Globally, the development of economic activities is associated with a great interest in energy-consuming services and the subsequent increase of fuel consumption. However, this fuel demand is commonly followed by high and unregulated carbon dioxide emission, which is the main source generator of the greenhouse gas (GHG) effect. Fossil fuels (mainly in the forms of petroleum, natural gas and coal) are experiencing accelerated consumption and stock depletion, despite meeting today’s global energy demand. Such combustion products are the main contributors to environmental problems and uncontrollable climate changes, thus threatening the global environmental safety and sustainability. Among feasible and realistic solutions to these global problems are those which have been proposed by engineers and scientists who agree to replace the existing fossil-fueled energy systems by the hydrogen energy system (HES). Subsequently, hydrogen can be fed to fuel energy-consuming services in order to improve energy security and simultaneously control the GHG effect
[1][2][3][1,2,3]. A plausible hydrogen energy system, which includes its resources, production technologies, storage, fuel-tank, dispensing and utilization, can be primarily analyzed for urban services, but it remains underdeveloped or sparsely studied in alignment with the renewable energy sources (RES). Hydrogen can be characterized as an efficient and clean fuel. Technological advantages are that the hydrogen combustion is neither a GHG producer nor a generator of ozone-layer-depleting chemicals. Moreover, hydrogen combustion does not generate acid rain ingredients or air pollution. It is also noteworthy that hydrogen produced from RES can develop a stable and permanent energy system that is never subject to future changes or modular modifications.
2. Hydrogen Applications
2.1. HES–RES in Practice
When approaching the technologies involved in hydrogen production from RES, besides the apparent environmental advantages of a highly efficient and clean fuel (no GHG contributor, no chemicals causing ozone depletion), it cannot be ignored that the RES technologies (e.g., solar, wind) are leading to permanent and fixed energy systems that cannot later change to include other RES types. In this context, the results developed above are suitable and significant for a wide spectrum of literature-oriented approaches toward RES utility among various HES. Among these, the electrifying transportation sector, which is a promising approach to alleviating climate change issues arising from increased emissions, is noteworthy
[4][71]. In this study, the examined HES can produce hydrogen, using RES, for the transportation sector (in buses). In this case, the electricity demanded for hydrogen production is harvested from the electrolysis of water, covered by RES. Moreover, fuel cells can use hydrogen to power the bus, while an HES exergy analysis referred to a steady-state model of the processes for which exergy efficiencies were calculated for all subsystems. Therefore, those subsystems showing the highest proportion of irreversibility were identified and compared. An exergetic efficiency of 12.74% for the PV panel, 45% for the wind turbine, 67% for the electrolysis, and 40% for the fuel cells was reported
[4][71].
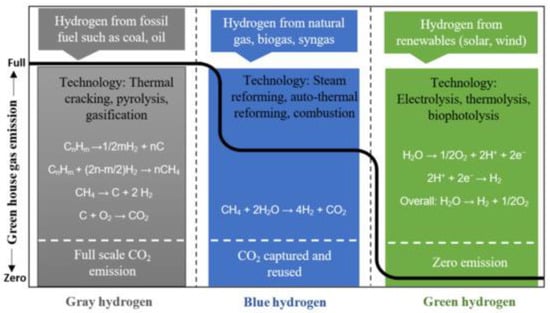
Depending on the production process and the selected (energy planning-based) energy source, the obtained hydrogen can be classified as grey, blue, or green, as shown in
Figure 12.
Figure 12. Outline of hydrogen-based feedstocks and technologies. Source: Sarker et al. [5]. Outline of hydrogen-based feedstocks and technologies. Source: Sarker et al. [72].
As shown in
Figure 12, the grey-hydrogen type is hydrogen produced from steam methane reforming and thermal cracking
[5][72]. As is also shown in
Figure 12, the main CO
2 production is reported through methane steam reforming, which can be further collected and stored safely in containers in the form of CO
2 vapors. Natural gas, biogas, and syngas can also produce hydrogen that is categorized as blue hydrogen, where the CO
2 gases formed cannot be stored, and will be released into the atmosphere. Blue hydrogen, which is produced from natural gas, unlike grey hydrogen, is capable of significantly reducing CO
2 emissions and simultaneously capturing and reusing carbon. As observed in
Figure 12, both grey- and blue-hydrogen production processes generate CO
2 as the by-product, but in the case of green-hydrogen production technologies, zero carbon emissions are also apparent. While solar and wind technologies have been utilized to produce green hydrogen, there are other catalytic-reforming technologies of sound capability for producing green hydrogen. Among them, biomass gasification and nuclear thermal/chemical pathways can potentially reduce carbon emissions. Because of this, major challenges such as production technology costs, system durability, reliability, infrastructure, and safety are issues of consideration
[5][72]. It is roughly estimated from the life-cycle assessment that hydrogen production through biomass gasification contributes less GHG emission (405–896.61 g CO
2/kg H
2) compared to wind-driven electrolysis (600–970 g CO
2/kg H
2)
[5][72].
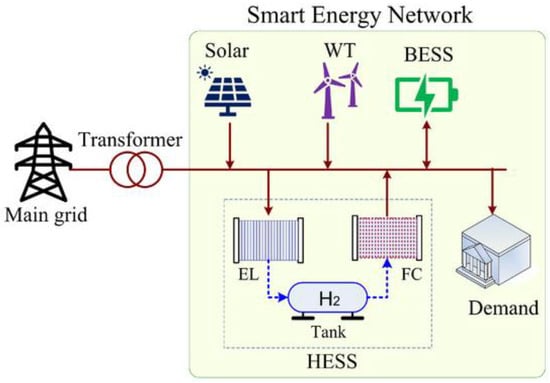
The schematic of the solar–hydrogen hybrid storage system (HESS) for the case study of a house is given in
Figure 23. The experimental structure of such an analysis considers a house which is remote from the national power grid with a meteorological solar/wind measurement tower in order to collect data used in the relevant case study. The meteorological measurement system can collect 10 min of data for average solar irradiance, solar duration, wind speed and direction, air temperature and pressure and also relative humidity. The desired characteristics of such an installation are devoted to providing uninterrupted power to houses at any time of the year through the suitable solar-HES.
Figure 23. A schematic of a typical domestic solar–hydrogen hybrid energy system. Source: Samende et al. [6]. A schematic of a typical domestic solar–hydrogen hybrid energy system. Source: Samende et al. [73].
A diagrammatic structure of a wind–hydrogen energy system, with its elements and some of their characteristics and relationships, are all shown in
Figure 23. The projected HESS can transform the energy into a storable product, hydrogen
[7][32]. This transformation process uses water electrolysis and the compression of hydrogen for storing. A technical limitation of the hydrogen tank is its finite capacity. Moreover, the latest transformation process is reforming hydrogen into ready-to-be distributed and sold energy. The pricing of energy selling is related to the energy-storage system through which energy can be sold when prices rise due to a demand increase
[7][32].
Besides the aforementioned constraint of finite storage of the hydrogen tank, other operational constraints of different system elements are the existence of a maximum capacity for transforming the energy into hydrogen, as well as a finite power limit, that is, a maximum amount of energy that can be dumped into the grid (and then sold) for each time unit. Another limitation is the inefficiency in the processes of transformation and recovery, since for these processes energy output is less than energy input
[7][32]. Because of this, modelling optimization is needed to determine the proper hydrogen storage capacity for the system’s optimization. When selecting from among different tank-size situations, it is noteworthy that storage tank capacity (a) is needed to transform the energy curve into a constant curve through time, and (b) is needed for storage of energy that has been produced above the power limit to dump it into the network again when the production is below the power limit
[7][32].
For an HESS, the simulation design is a key factor, taking into consideration also the constraint of missing data for continuous yearly consumption measurements, whereas measurements are subject to daily and seasonal variations of power consumption in houses. Indeed, energy consumption decreases, since the households are at sleep from sunset and midnight and up until the next morning, where the fuel cell stack is the only available power source in the hybrid system. Regarding seasonal variation, higher energy consumption is reported in winter (the most in-house occupation) than in summer
[6][73]. Regarding the HESS, it is noted that the hydrogen amount in the storage tank decreases from November to February, followed by an increase from March to May, thus shaping repeating hydrogen cycles (second-year cycles) with an arrangement of solar panels and some decades of the cubic meters of storage tanks given.
2.2. HES and Electrolyzer Efficiency
In the relevant literature
[8][49], the general process that researchers follow in order to understand in a comprehensive way the effects of the space between the two electrodes on the rate of hydrogen production, among the different gaps examined, was demonstrated. Loss in mass increase is observed with voltage increase, under the condition of a constant space between the two electrodes. The resulting increase in the percentage mass lost due to the increase in the voltage is attributed to the increase in electrical current. During relevant experimental tests, the pair of electrodes can be exposed to a PV generator with low voltages of 2–5 V. When testing different concentrations of electrolyte solution, then, the percentage water mass lost during a specified time is measurable and the corresponding electrolyzer efficiency is calculated using the mass ratio of the hydrogen in a single molar mass of water
[8][49]. Subsequently, voltage input and the gap between the electrodes play a determining role in the overall performance of the water electrolysis unit. Higher hydrogen production rates are achievable at a closer space between the electrodes, as well as at higher voltage input. Maximum electrolyzer efficiency is realistic for a smaller gap between electrodes, coupled with a specified input voltage value within the designed range
[8][49]. A decrease in space between the pair of electrodes results in lowering the percentage of water mass loss, which increases due to the resulting decrease in electrical resistance between the electrodes, and thus leads to an increase in the electrical current. Hydrogen production rate increase, and thus energy efficiency increase, is due to the decrease in the space between the electrodes (small gaps between the pair of electrodes) being noticeable at higher degrees of input voltage, up to a specified input voltage; then, efficiency decreases by the further increase in the input voltage
[8][49].
Figure 34 illustrates the schematic flow chart for hydrogen production.
Figure 34. An overview of RES-based hydrogen hybrid system. Source: Sarker et al. [5]. An overview of RES-based hydrogen hybrid system. Source: Sarker et al. [72].
As depicted in
Figure 34, above, the RES-based hydrogen hybrid system, which in the literature is referred to also as a hybrid renewable energy system (HRES), has proven a feasible solution to address the issues related to individual energy sources
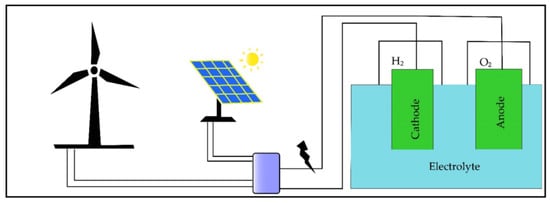
[5][72]. Usually, a typical hybrid green-energy system uses various RES, such as wind and solar, as shown in
Figure 45.
Figure 45. Hydrogen production from hybrid energy-based electrolysis. Source: Sarker et al. [5]. Hydrogen production from hybrid energy-based electrolysis. Source: Sarker et al. [72].
The benefits of HRES rely on multiple RES that are able to supply consistent and uninterruptible energy. Therefore, this energy availability will compensate for the unreliability of single renewable-energy sources and reduce GHG emissions
[5][72]. It is also noteworthy that such systems are mainly located very close to the place of demand, thus lowering the possibility of damage to the transmission wire, and supporting also a prompt access to repair and maintenance when needed.
2.3. Hybrid HES-RES Aspects
Hence, hydrogen storage systems should incorporate more compact, more robust and less-costly fuel cell systems, being installed before wind/hydrogen systems. Additionally, multiple-energy-fed hybrid system solutions—such as wind, solar, and bioenergy—should be selected according to the spatial RES characteristics of each location. Moreover, HES and RES projects should be designed in alignment with “green” incentives, green certificates, and CO
2 tax-free policies. Other economics incentives are the fluctuation in oil and gas prices, the capital monetization of green energy infrastructure and the energy supply security. These factors justify the construction and motivate the development of a full-scale demonstration system (such as the wind/hydrogen plant at Utsira). In this case, such types of demonstrations can plausibly promote public awareness and acceptance, improving RES cost competitiveness, and regulating the new energy-market barriers and the enablers of new technological solutions (in general) and hydrogen technology (in particular)
[5][72].
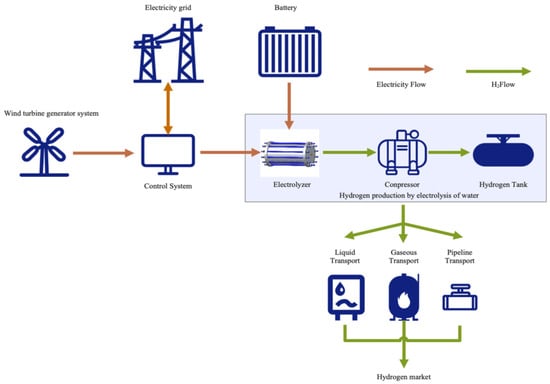
A typical integrated modular representation of widely used hydrogen-based hybrid systems is depicted in
Figure 56.
Figure 56. Schematic flow chart of a wind/hydrogen plant. Source: Niu et al. [9]. Schematic flow chart of a wind/hydrogen plant. Source: Niu et al. [74].
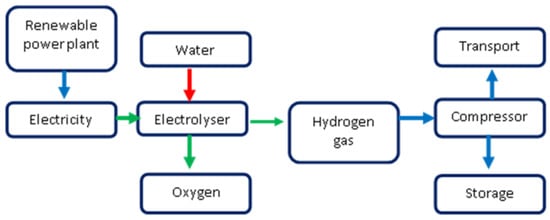
The system integrates various elements, including renewable energy generation, power storage, hydrogen production, and hydrogen transportation (
Figure 45). The electricity generated by a wind turbine can be directly supplied to the power grid or stored in batteries for future use, optimized by the control system. Another fraction of the electricity is utilized in an electrolyzer device, which separates water (H
2O) into hydrogen gas (H
2) and oxygen gas (O
2). The generated hydrogen gas is then compressed and stored in hydrogen tanks for further transportation and utilization
[9][74]. In this process, the electrolyzer, compressor, and hydrogen tanks are essential components for hydrogen production through water electrolysis. Hydrogen gas can be transported to various demand points, being accessible to the hydrogen market through various methods, such as liquid transportation, gas transportation, or pipeline transportation. Liquid transportation is suitable for long-distance transport, while gas and pipeline transportation are commonly used for short-distance distribution. In this context, the term “Electricity Flow” (
Figure 56) refers to the movement of electricity from the wind turbine through the control system to the power grid, batteries, and electrolyzer. Similarly, the term “H
2 Flow” (
Figure 56) represents the process of hydrogen gas being produced by the electrolyzer, compressed, stored, and ultimately transported to the hydrogen market
[9][74].
Based on the configuration of
Figure 56, it can be seen that the PV operating voltage and current determine the power output of the PV array and depend on the insolation on the PV module, the ambient temperature, and the manufacture characteristics of the PV module. Such a successful operation system for the wind turbine unit depends on several climatic and technical factors, such as wind speed and the rated cut-out and cut-in speeds (in m/s) for the wind turbine
[10][26]. From a design perspective, the optimal design for off-grid hybrid renewable systems based on solar and wind energy to continuously meet the load necessitates a consideration of the LPSP (loss of power supply probability) and minimizing of the TLCC (total life-cycle cost), subject to constraints. The optimization algorithm is the most determining factor that can be efficiently used for this type of designed hybrid energy system. In particular, at low LPSP values (0–5%), the combination of a hydrogen and photovoltaic (PV) scheme with weather forecasting data leads to the most cost-effective system, and at LPSP = 10% the combination of wind, PV and a hydrogen scheme is proven to be the most cost-effective hybrid system
[10][26].
In a similar optimization study of a stand-alone renewable H
2-based microgrid, its optimal sizing requires a reliable load demand to satisfy, by means of local renewable energy supported by a hybrid battery/hydrogen storage unit, the minimization of the system costs. A crucial factor is the price reduction, due to the installation and operation of a high number of components. In similar studies, the development and application of a mixed-integer linear programming technique (MILP) optimization framework to an off-grid village in Italy was reported, which is a typical insular case study of the Mediterranean area
[11][21]. To model the seasonal storage, a year-long time horizon was considered necessary for off-grid areas in order to achieve energy independence by relying on local RES. The degradation costs of batteries and H
2-based devices were included in the objective function of the optimization problem, such as the annual cost of the system. Efficiency and investment-cost curves were considered for the electrolyzer and fuel cell components, while the design optimization was also performed while employing a general demand response program (DRP) to assess the sizing effects on the whole performance.
2.4. HES-RES and Safety Aspects
In HES, hydrogen is an intermediary or secondary form of energy or an energy carrier. Hydrogen complements the primary energy sources, putting them into a convenient form accessible to the desired locations and at times convenient for consumers. Hydrogen can be stored underground in ex-mines, caverns and/or aquifers, which is of utmost importance for large-scale storage cases. The main safety aspects of the HES operation refer to hydrogen re-electrification. Indeed, hydrogen re-electrification refers to electricity generation from hydrogen. Through combustion, hydrogen can be re-electrified. Some combustion engines or turbines can run directly on hydrogen, similarly to internal combustion engines running on gasoline. Nevertheless, comparing the efficiencies of hydrogen and gasoline combustion engines, the former performed less efficiently than the latter, having a thermodynamic efficiency of around 20–25%, since hydrogen has a relatively low volumetric energy density. Moreover, while no CO
2 is released, through hydrogen combustion NOx are emitted. The fuel cell-fed engines can be proven advantageous for hydrogen, as fuel cells convert the hydrogen chemical energy directly into electrical energy, achieving almost 60–80% efficiency, having as a byproduct only water
[12][58]. The wide commercial applicability of fuel cells in various stationary and transportation applications can be stressed
[12][58].
The exact procedure is the following: hydrogen transportation is occurs through pipelines or super tankers, making it directly disposable to energy consumption centers. Subsequently, specific applications regarding the electricity and transportation, and industrial, residential and commercial uses such as a fuel and/or an energy carrier, take place. Regarding the water production, recycling of water and water vapor follows when these effluents are disposed through rain, rivers, lakes and oceans, making up for the water used in the first place to manufacture hydrogen
[13][70].
Regarding the safety aspects of hydrogen, these involve its toxicity on one hand and its fire hazard properties on the other. In relation to this, regarding the toxicity of hydrogen combustion products, the toxicity increases as the carbon-to-hydrogen ratio increases. For instance, hydrogen and its main combustion product, water or water vapor, are not toxic, but NOx, which can be produced through the flame combustion of hydrogen (also reported in fossil fuel combustion) displays toxic effects
[13][70]. In general, higher specific heat causes a gaseous fuel to be safer, since it slows down the temperature increases for a given heat input. Wider ignition limits, lower ignition energies, and lower ignition temperatures make gaseous fuels less safe, as they increase the limits at which a fire could commence. Higher flame temperature, higher explosion energy, and higher flame emissivity all make gaseous fuels less safe as well, due to the damaging fire consequences
[13][70]. Extra safety concerns and provisions have to be prioritized in the design of in-site plants such as the Utsira plant
[14][75].
2.5. HES-RES and Techno-Economic Aspects
As hydrogen can be further used in internal combustion engines that are designed similarly to traditional combustion engines, it can be pointed out that both the mature industry and the vast availability of production infrastructure regarding internal combustion engines are making hydrogen internal combustion engines economically attractive. Moreover, unlike fuel cell vehicles, these types of vehicles do not rely on materials that may limit their large-scale production
[15][63]. These engines support different characteristics, such as traditional gasoline engines that utilize electronic control units for the proper pressure management in the injection and in the hydrogen combustor. In addition, these engines mainly emit nitrogen mixtures, and thus they are not emission free
[15][63].
In economic terms, the increase in pressure in the hydrogen storage from 200 bar to 450 bar, or even 700 bar, would increase the overall energy density of the hydrogen storage, thus making it possible to store more wind energy on the same footprint. However, high-pressure hydrogen storage systems are likely to be more costly than low-pressure systems, both from an investment and an operational point of view. It is noteworthy that investment cost increase is mainly linked to the installment of stronger storage tanks (thicker steel walls and/or use of composite materials), while operational cost increase is mainly linked to the increase in energy consumption for hydrogen compression
[14][75]. It is also likely that extra costs associated with the required safety system concur, being dependent on the type of system installed. because of this, two basic techniques for high-pressure hydrogen gas production are: (a) low-pressure electrolysis, with a long compression stage, or (b) high-pressure electrolysis without compression
[14][75].
While the majority of research studies have been focused on the HES-RES coordination, it is noteworthy that there are also reported RES studies, other than those of solar- and wind-energy infrastructure for hydrogen production. Among these studies, wastewater was characterized as an issue of general concern for environmental sustainability, making the development of a circular low-waste economy a necessity. In this context, lignocellulosic biomass processing (mainly hydrolysis, pyrolysis, and hydrothermal liquefaction) can result in secondary aqueous streams in which there are low quantities of carbon and biomass, making the conventional valorization treatment complicated. In this respect, biodiesel production leads to a glycerol excess on the market, which needs to be valorized
[16][76].
2.6. HES-RES and Environmental Aspects
As already mentioned above, hydrogen utilization involves oxidation, and the only direct major product from the hydrogen oxidation is water. Small quantities of nitrogen oxides are released when hydrogen is combusted in air, but these effluents can be controlled with careful engine design
[15][63]. The environmental impacts from other phases in the life cycle of a hydrogen system are similar to those for other energy technologies, and may be small or large, depending on the source of the hydrogen
[15][63]. Regarding the environmental concerns and considerations, an HES is prone to cause direct and indirect environmental (especially atmospheric) impacts, at three steps: production, transportation-storage and utilization. At the production step: the leaking of hydrogen and carbon dioxide release in the case of production from fossil fuels; at the transport–storage steps: the leaking of hydrogen; and at the utilization step: the release of water and the leaking of hydrogen. On the basis of experience with technologies associated with the transportation of natural gas and other volatiles, it seems likely that systems of hydrogen production, storage, and transport will involve losses to the atmosphere.
At this point, it can be seen that human activity can result in approximate duplication or triplication of the scheduled annual production of hydrogen from all sources combined. In the case of replacing all fossil-fuel energy generation with hydrogen fuel cells, then an amount of approximately 60 million tons annually of human-made hydrogen would leak into the atmosphere: this is roughly four times the current amount. Subsequently, such hydrogen leaks might increase water vapor and cool the stratosphere, through retarding the ozone-layer recovery. This excessive release of hydrogen into the atmosphere is also worsening, due to natural sources of hydrogen
[17][64]. Furthermore, hydrogen participates in stratospheric chemical cycles of water and various GHGs, where this substantial increase in its concentration can cause irreversible changes in the stratosphere regarding the imbalance of equilibrium in the concentration of its constituent components. High amounts of hydrogen emissions are also unavoidable from a global fleet of fuel cell vehicles, further impacting on local or regional distribution of water vapor. Water vapor increase can also affect local, regional, and global climatic conditions, mainly due to an increase in relative humidity among areas of widely applied fuel cell technologies, compared to the operation of internal combustion engines.
2.7. Miscellaneous Aspects
Other critical design aspects of hydrogen utility in combined sources of energy generation are the following
[18][33]:
- –
-
The selection of the number of fuel cell stacks should consider the price increase of additional fuel cells with the decrease in the price of the smaller storage tank.
-
- –
-
An analysis of the solar–hydrogen hybrid system should consider the effect of the solar irradiance and the ambient air temperature, which are key determinants for calculating the power from the solar panel array. Those reliable and uninterrupted systems of energy are depended on the number of solar panels used in the system. The number of solar panels used in the system affects the electrolyzer size as well as the storage tank volume.
-
- –
-
The efficiency of the hybrid system is dependent on the nominal electrolyzer power. Proper sizing of the electrolyzer can reduce the non-utilizable energy and therefore increase efficiency. The nominal power of the electrolyzer also determines the number of solar panels and the storage tank volume.
-
- –
-
Modular designers cannot ignore the fact that simulations have to consider short time intervals for constructing the properly sized components in the system; otherwise, there will be inevitable interruptions in the power from time to time with undersized hybrid system components.
-
Other researchers proposed the following design, aspects of hydrogen utility in combined sources of energy generation
[13][70]:
- –
-
Liquid and gaseous hydrogen are valued as the best transportation fuels when compared to liquid fuels such as gasoline, jet fuel and alcohols.
-
- –
-
Hydrogen is a versatile fuel that can be converted to useful thermal-, mechanical- and electrical-energy forms for end-users through a variety of processes, whereas fossil fuels can only be converted through one process, i.e., flame combustion.
-
- –
-
In quantitative terms, hydrogen is 39% more efficient than fossil fuels. Moreover, hydrogen is an energy-conserving fuel that can save primary energy resources.
-
- –
-
In safety terms, hydrogen is safe for use, avoiding fire hazard and toxicity cases.
-
Moreover, the design aspects cannot ignore the selection of the site for the HES installation. Indeed, an appropriate location has to support the following features: good-to-excellent wind conditions, small but representative load, back-up systems in place, not too remote, a supporting community, and access to service personnel. In general, all equipment should be kept as simple and robust as possible, and redundancy should be considered. Due to the uncertainty in precisely forecasting wind power production and customer power demand, a slightly oversized installation should also be considered. However, there is a tradeoff to be made between plant availability and overall system cost
[14][75].
3. Conclusions
It is important to follow a logical sequence of actions: (a) the design of an optimal hydrogen supply system, (b) the evaluation of its environmental impacts, and (c) an understanding of the effects of various factors that aid the selection and the installation of an optimal hydrogen supply system through a scenario plan and sensitivity analysis. A research limitation of this approach is the inability to directly foresee the actual magnitude of hydrogen emissions associated with a hydrogen fuel cell economy, particularly since today budgets for hydrogen are not fully or well known, while there are also technical constraints, and the future fuel cell industry can be only forecasted. In this case, the evolution and the shift from a fossil-fuel combustion energy planning to the prevalence of hydrogen fuel cells can actually result in unpleasant anthropocentric hydrogen emissions, because fossil fuel combustion is a source of hydrogen itself. On the other hand, researchers are deemed to take into account the climatic effects of HES in the near-future energy plans, especially those based on electrolysis from water, where the simultaneous reduction of fossil-fuel emissions must be also considered [17][64].
 Encyclopedia
Encyclopedia
