Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zhifeng Wang and Version 2 by Lindsay Dong.
Ubiquitin-fold modifier 1 (UFM1) is a newly identified ubiquitin-like protein that has been conserved during the evolution of multicellular organisms. In a similar manner to ubiquitin, UFM1 can become covalently linked to the lysine residue of a substrate via a dedicated enzymatic cascade. Although a limited number of substrates have been identified so far, UFM1 modification (UFMylation) has been demonstrated to play a vital role in a variety of cellular activities, including mammalian development, ribosome biogenesis, the DNA damage response, endoplasmic reticulum stress responses, immune responses, and tumorigenesis.
- post-translational modification
- UFMylation
- ubiquitin-like proteins
1. Introduction
Post-translational modification (PTM) refers to the addition of chemical groups to one or more amino acid residues; such additions can substantially change the biological activity of the target protein [1]. To date, more than 500 different protein PTMs have been identified, including phosphorylation, glycosylation, acetylation, and ubiquitination [2]. Focusing on the latter, ubiquitin (Ub) is a small protein weighing approximately 8.5 kDa and comprising 76 amino acids. Ub is widely distributed in all eukaryotic cells and has been highly conserved during evolution. Indeed, yeast and human ubiquitin differ by only three amino acids. Ubiquitination refers to the covalent binding of Ub to target proteins, and generally requires the synergistic action of three ubiquitinating enzymes: E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin-ligase [3]. First, ubiquitin is activated by E1 using energy provided by ATP hydrolysis, then transferred to E2, and, finally, covalently linked to the lysine residues of substrates with the aid of E3. Ubiquitination is a tightly regulated and reversible process: deubiquitinating enzymes (DUBs) can reverse ubiquitination by hydrolyzing the peptide or isopeptide bonds between ubiquitin molecules or between ubiquitin and substrate proteins [3]. Ubiquitination helps to regulate numerous biological processes, encompassing immune responses [4][5][4,5], the DNA damage response [6][7][6,7], cell cycle regulation [8], autophagy [9], epigenetic modulation [10], cellular apoptosis [11], and protein degradation [12], by regulating protein structures, interactions, activities, and even subcellular localizations [3].
The many ubiquitin-like proteins (UBLs) identified to date include small ubiquitin-like modifiers (SUMOs), neural precursor cell-expressed developmentally downregulated 8 (NEDD8), and interferon-stimulated gene 15 (ISG15). Although most UBLs do not necessarily share notable sequence homology with Ub, they all share a similar tertiary structure [13]. Similar to Ub, UBLs can covalently bind to target proteins (UBLylation) through a series of enzymatic reactions, similar to those involved in ubiquitination, to confer different biological functions to the substrate [14].
Ubiquitin-fold modifier 1 (UFM1) is a novel UBL formed from an 85-amino acid precursor (pro-UFM1) that is translated in human cells. UFM1 is evolutionarily conserved in multicellular organisms, but is absent in yeast [15]. Similar to Ub, the C-terminal serine and cysteine of the UFM1 precursor can be removed using specific proteases to expose the C-terminal glycine, leaving mature UFM1 to directly and covalently attach to the lysine residues of target substrates [16]. UFM1 is a unique UBL as it has only one glycine residue at its C-terminus.
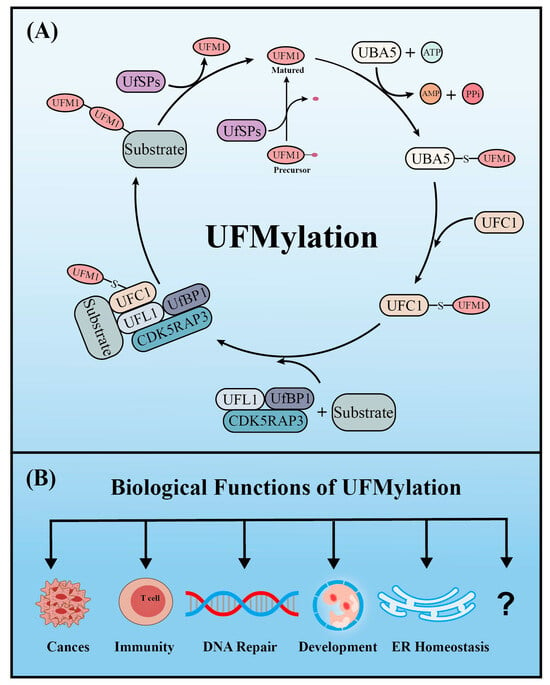
2. UFMylation
Similar to ubiquitination, UFM1 covalently binds to its target proteins through a cascade involving three enzymes. As described above, the UFM1 gene is translated into a precursor form and the C-terminal glycine must be exposed by UFM1-specific proteases (UfSPs) before subsequent enzymatic reactions can occur [16]. First, when ATP is present, the E1-like enzyme UBA5 activates the mature UFM1, which forms a high-energy thioester bond between the catalytic cysteine (Cys250) of UBA5 and the exposed C-terminal glycine of UFM1. Next, the E2-like enzyme UFC1 interacts with the UFC1 binding domain of UBA5, which transfers the activated UFM1 to UFC1 by forming a similar thioester bond between UFM1 and the catalytic cysteine (Cys 116) of UFC1. Finally, UFM1 is coupled to the lysine residues of its target proteins in a process mediated by the E3-like enzyme UFL1 [17][18]. Another similarity with ubiquitination is that UFMylation is also reversible. In addition to maturing UFM1, UfSPs also cleave UFM1 from its target protein, thereby rendering UFM1 and its substrates recyclable (Figure 1) [16]. Numerous verified substrates of UFM1 have been discovered and the impact of UFMylation on their functions elucidated.
Figure 1.
The enzyme cascade of UFMylation (
A
) and five key biological functions of UFMylation (
B
).
2.1. UfSPs
Pro-UFM1 maturation and the removal of UFMylation from substrates are mediated by the cysteine proteases UfSP1 and UfSP2, respectively. It was, until recently, thought that UfSP2 was the only active protease because UfSP1 apparently lacks a catalytic domain [16]. However, two independent groups recently found that UfSP1 actually utilizes a non-canonical start codon (217CUG) upstream of its canonical counterpart (445AUG) to initiate translation, which produces a catalytically active UfSP1. Cong et al. reported that both UfSP1 and UfSP2 can mediate the maturation of pro-UFM1 and the de-UFMylation of substrate proteins [18][34]. By contrast, a study by Kulathu et al.’s group indicated that UfSP2, but not UfSP1, de-UFMylates the ribosomal subunit RPL26, while UfSP1 removes the constitutively autoinhibitory UFMylation of UFC1, thereby promoting the activation of UFMylation [19][20].
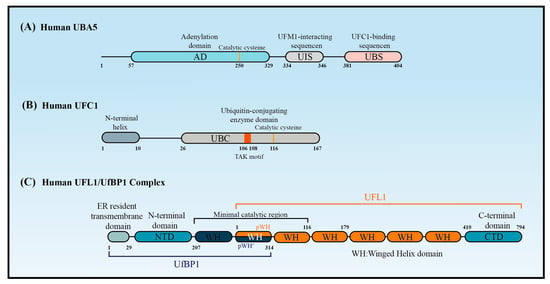
2.2. UBA5
The canonical E1 enzymes that mediate ubiquitination have conserved adenylation domains, catalytic cysteine domains, and ubiquitin-fold domains. By contrast, as a member of the non-canonical E1 enzyme family, UBA5 does not contain a catalytic cysteine domain. Instead, its catalytic cysteine active site (Cys250) is located in its adenylation domain [20][37] (Figure 2A). This domain comprises an eight-stranded beta sheet that is surrounded by helices [21][38] and it promotes UBA5 homodimer formation with pseudo-two-fold symmetry [22][39]. Short and long UBA5 are two distinct isoforms of UBA5 encoded by the human genome, with the latter distinguished by the presence of a 56-amino acid extension at the N-terminal adjacent to the adenylation domain. Structural and biochemical studies indicated that the binding ratio of UBA5 to ATP in the presence of the N-terminus (long UBA5) is 1:1 rather than the 2:1 that occurs in its absence (short UBA5). This finding indicates that the N-terminus greatly increases the affinity of UBA5 for ATP, thereby promoting UFM1 activation at low ATP concentrations [23][40]. The N-terminal extension also enhances the thermal stability of UBA5 and promotes the faster transfer of UFM1 to UFC1 through a conformational change occurring at the N-terminus of UBA5 when ATP binds to the UBA5-UFM1 complex. Therefore, ATP and the binding of UFM1 to UBA5 stabilize the UBA5 homodimer, enhance UBA5 stability, and promote UFM1 transfer to UFC1.2.3. UFC1
UFC1 is mainly localized in the nucleus, with a minor proportion located in the cytoplasm [15]. UFC1 lacks some features that are conserved in other E2s, such as the catalytic histidine–proline–asparagine (HPN) motif, which suggests it has a unique mechanism of function and modulation [24][45]. Some ubiquitin E2 enzymes bind to Ub to promote E2 dimerization, stabilize E2s in their active-closed state, and enhance their catalytic efficiency [3]. It is unknown whether UFC1 activity is regulated by similar mechanisms. Although UFC1 differs from the other E2 enzymes, it contains a catalytic core structural domain that is conserved in all E2 enzymes. This catalytic core domain presents as a flexible loop formed of approximately 10 amino acid residues and confers strong solvent accessibility [25][46]. The loop encloses the intermediate active cysteine residue (Cys116), which undergoes a trans-esterification reaction upon the transfer of UFM1 from UBA5 to UFC1 [25][46] (Figure 2B). UFC1 may transfer UFM1 to substrates in association with UFL1/UfBP1. Data from in vitro assays confirmed that UFC1 can transfer UFM1 to free cysteines but not free lysines, indicating that UFC1 cannot transfer UFM1 to substrates directly. UFC1 together with UFL1/UfBP1 can, however, transfer UFM1 to free lysines [26][47].2.4. UFL1 and UfBP1
UFL1 contains a transmembrane domain enabling its primary localization on the cytoplasmic side of the endoplasmic reticulum (ER) membrane. UFL1 can, however, also be found in the cytoplasm and nucleus due to the presence of a nuclear localization signal [27][48]. In the UFMylation system, UFL1 is the only E3 ligase identified so far that aids the transfer of UFM1 to target substrates [28][49]. Hundreds of E3 ligases that participate in ubiquitination are classified into three types according to domain structures. RING E3s contain the very interesting new gene domain, while HECT E3s contain a domain that is homologous to the E6-AP C-terminus domain, and RBR E3s contain a RING-between-RING domain. RING E3s transfer ubiquitin directly from the E2s to the substrates without binding to the Ub, while HECT and RBR E3s require Ub to first form a thioester bond with the conserved cysteine before the Ub is transferred to the substrates [29][50]. Interestingly, UFL1 cannot function properly alone, but requires UfBP1 to promote its stability and activity. UfBP1, also known as C20orf116 or DDRGK1, is a UFM1-interacting protein composed of 314 amino acids that include a C-terminal proteasome-COP9-initiation factor domain. UfBP1 also contains a transmembrane domain and is localized on the cytoplasmic side of the ER membrane, where it exists in a complex with UFL1 [30][19]. Structure predictions have shown that UFL1 and UfBP1 form a heterodimer composed of several winged helix (WH) domain repeats [26][47] (Figure 2C). UFL1 has an N-terminal helix followed by a partial WH (pWH) and five WH domains, which are important for its E3 ligase activity. UfBP1 has an N-terminal transmembrane segment, a long helical region followed by a WH (WH1′) domain, and a partial WH (pWH′) domain [31][33]. The partial pWH domain at the N-terminus of UFL1 complements the partial pWH′ domain at the C-terminus of UfBP1 to form a composite WH (pWH-pWH′) domain that is essential for complex formation and protein stability [31][33]. CDK5RAP3 is another protein that is consistently associated with UFL1. Because CDK5RAP3 always functions as a substrate adaptor [32][53], it is thought that CDK5RAP3, together with UFL1/UfBP1, forms part of an integral E3 ligase complex [33][34][35][51,54,55]. CDK5RAP3 binds to the ligase complex of UFL1/UfBP1 and restricts its E3 ligase activity. CDK5RAP3 functions as a specificity determinant, inhibiting ligase activity in the absence of a substrate and directing ligase activity toward the ribosomal subunit RPL26 [26][47].
Figure 2. The structure of UBA5, UFC1, and UFL1/UfBP1 complex. Schematic of (A) the key domains of UBA5, (B) the key domain features of UFC1, and (C) the domains of the UFL1-UfBP1 E3 ligase complex.
3. Biological Functions of UFMylation
3.1. UFMylation and the DNA Damage Response
DNA damage caused by endogenous or exogenous factors seriously impairs genomic integrity but can be rescued via DNA damage response pathways [36][56]. DNA double-strand breaks (DSBs), which are extremely toxic to cells, are repaired almost exclusively by homologous recombination (HR) and non-homologous end-joining. Emerging evidence indicates that UFMylation has numerous important roles in mediating the cellular response to DSBs, thus contributing to the maintenance of genome stability and preventing tumorigenesis [37][57].3.1.1. MER11 UFMylation
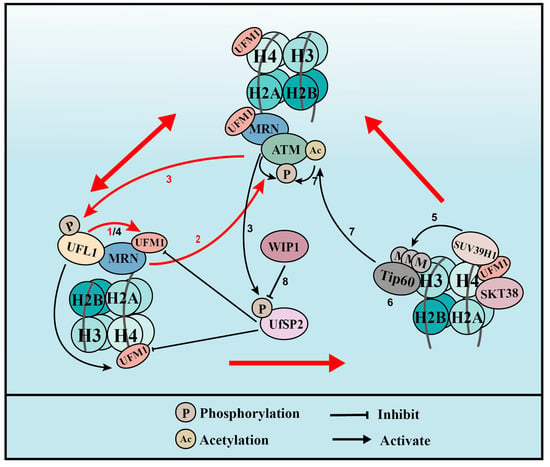
UFL1 co-localizes with γH2AX, a marker of DSBs, during UV- and IR-induced DNA damage. In addition, UFM1 and UFL1 are immediately recruited to laser-induced DSBs [38][39][25,26]. These observations have prompted us to speculate that UFMylation has a role in the DSB response. At the initiation of the DSB response, the MRE11-RAD50-NBS1 (MRN) complex re-localizes to the damage sites in what is regarded as the first and most important step that activates the kinase of ataxia telangiectasia mutated (ATM) [36][56]. UFL1 depletion inhibited the activation of ATM after DSB formation [37][57], indicating that UFMylation plays important roles in ATM activation. The interaction between UFL1 and the MRN complex, together with the decreased recruitment and stability of the MRN complex resulting from UFL1 depletion, led to the identification of MRE11 UFMylation on K282 [37][38][25,57]. MRE11 is a core factor in the MRN complex and binds directly to RAD50 and NBS1. This complex is integrally recruited to damage sites, which promotes ATM activation and DNA end resection, thereby promoting HR repair [36][56]. The function of MRE11 UFMylation in the DSB response is also mediated by UfSP2 [40][58]. Evidence from human cell lines showed that UfSP2 is phosphorylated at serine 374/381 by ATM, which promotes its release from the MRN complex after the formation of DSBs [38][40][25,58]. This release enhances MRE11 UFMylation and ATM activation, which, in turn, further promotes UfSP2 phosphorylation. UfSP2 is dephosphorylated by the phosphatase WIP1, which promotes UfSP2 recruitment to DSBs, leading to the de-UFMylation of MRE11 and H4, and suppresses ATM activation [40][58].3.1.2. Histone H4 UFMylation
Another mechanism by which UFMylation regulates DSB-induced ATM activation involves UFL1 phosphorylation at serine 462 by ATM. This event promotes UFL1 recruitment to sites of DNA damage. UFL1 catalyzes the mono-UFMylation of histone H4 on K31 [39][26], which can be recognized by the UFMylation reader serine/threonine kinase 38 (STK38) [41][60]. STK38 contains a UFM1-binding motif that when mutated at conserved amino acids can no longer interact with UFMylated H4. STK38 recruitment to DNA damage sites also depends on the monoacylation of H4 and is critical for the subsequent recruitment of SUV39H1. SUV39H1 catalyzes the trimethylation of H3K9 (H3K9me3), which binds to and recruits Tip60. In turn, Tip60 acetylates ATM and promotes its activation [39][26]. The UFMylation of H4 is also regulated by UfSP2 and its ATM-induced phosphorylation (see MRE11 above). MRE11 UFMylation at DSBs is first catalyzed by UFL1 to promote MRN complex recruitment and ATM activation. Active ATM then phosphorylates UFL1 and UfSP2. The phosphorylation of UFL1 enhances its recruitment to sites of DNA damage, while the phosphorylation of UfSP2 releases it from the MRN complex, and both processes further increase MRE11 UFMylation. UFL1 and UfSP2 phosphorylation also leads to enhanced H4 UFMylation and, later, ATM activation. The phosphatase WIP1 de-phosphorylates UfSP2, which de-UFMylates MRE11 and H4, and finally reverses ATM activation. UFMylation therefore promotes ATM activation though two distinct positive feedback loops: one is WIP1-UFL1/UfSP2-MRE11-ATM-UFL1/UfSP2 phosphorylation and the other is WIP1-UFL1/UfSP2-H4-STK38-SUV39H1-H3K9me3-Tip60-ATM acetylation-ATM phosphorylation-UFL1/UfSP2 phosphorylation (Figure 3).
Figure 3. UFMylation of MRE11 and H4 promotes ATM activation (for the purposes of the diagram, MRE11 is abbreviated to MRN complex). (1) Upon DNA damage, UFL1 is recruited to chromatin via MRE11 to UFMylate MRE11, which promotes the formation and recruitment of the MRE11-RAD50-NBS1 (MRN) complex. (2) UFMylated MRE11, together with RAD50 and NBS1, activates ATM. (3) Activated ATM further phosphorylates UFL1 and UfSP2, which promotes UFL1 recruitment, while inhibiting UfSP2 recruitment. (4) As a positive feedback loop, phosphorylated UFL1 further enhances MRE11 UFMylation and UFMylates H4. (5) UFMylated H4 can be recognized by the UFMylation reader serine/threonine kinase 38 (STK38), which is critical for the subsequent recruitment of the methyltransferase SUV39H1. (6) SUV39H1 trimethylates lysine 9 of histone H3 (H3K9me3), which recruits Tip60. (7) Tip60 acetylates ataxia telangiectasia mutated (ATM) and promotes ATM activation. (8) Lastly, the phosphatase WIP1 de-phosphorylates UfSP2 and reverses ATM activation. Red arrows indicate the two positive feedback loops.
3.2. UFMylation in ER Metabolism
3.2.1. ER Stress
ER stress is caused by an overload of un/mis-folded proteins in the ER. In mammalian cells, three ER transmembrane proteins act as sensors of ER stress: activating transcription factor 6, inositol-required enzyme 1α (IRE1α), and PKR-like ER kinase (PERK) [42][61]. The accumulation of un/mis-folded proteins triggers ER stress and activates un/mis-folded-protein response (UPR) signaling to the ER to enhance its protein processing capacity. Therefore, the amount of UPR signaling is representative of ER stress levels. The un/mis-folded-proteins are then degraded through a type of ubiquitin-proteasome degradation known as ER-associated degradation (ERAD) [42][61]. Damaged ER can also be removed through an autophagy process called ER-phagy [43][62]. Thus, ERAD and ER-phagy are the predominant pathways involved in alleviating ER stress.
As mentioned above, UFL1 and UfBP1 are localized on the ER membrane, which suggests that UFMylation is involved in ER stress. The first evidence that UFMylation is related to ER stress was obtained in studies to determine the involvement of ER stress in the development of heart disease [44][63]. Later, numerous studies showed that the UFMylation substrate UfBP1, an UFL1 partner, could respond to ER stress [28][45][46][17,49,64], and CDK5RAP3 could sense proteotoxic stress in the ER lumen by forming a tripartite receptor complex with the ER-associated UFL1/UfBP1 [47][65]. In another example, cisplatin induced a stress-related increase in UFL1 expression in granulosa cells and enhanced ER stress, processes which were exacerbated by UFL1 knockdown and alleviated by UFL1 overexpression [48][66]. Others have reported that UFMylation modulates UPR signaling.
CDK5RAP3, another UFL1 partner, is also involved in ER stress, especially the expression of XBP1 and PERK [49][69]. During the interphase of the cell cycle, microtubules are predominantly nucleated at the centrosome (microtubule organizing centers; MTOC) by γ-tubulin ring complex (γTuRC) proteins. Most γTuRCs are activated by structural rearrangement, phosphorylation, or binding to modulating proteins accumulated in MTOCs [49][69]. The interaction of ER membranes with newly formed microtubules could promote ER expansion and help to restore ER homeostasis. UFL1 can interact with CDK5RAP3 and form a complex with γTuRCs [50][51][70,71], negatively regulating microtubule nucleation at interphase centrosomes. In mammalian cells, ER network rearrangements largely depend on interactions with dynamic microtubules. Therefore, an UFL1/CDK5RAP3 deletion induces ER stress and the release of γTuRC proteins, which in turn stimulates microtubule nucleation. Thus, the interaction between the ER and newly formed microtubules promotes ER enlargement to restore ER homeostasis.
